Building bones using stem cells and gene therapy
Dr Pascale V. Guillot is based at the University College London Elizabeth Garrett Anderson Institute for Women’s Health. She is leading a team of cell biologists who are using human stem cells to grow both healthy and brittle bone tissue in the lab. Why? So they can investigate the causes of a brittle bone disease called osteogenesis imperfecta and develop innovative treatments to make bones stronger
TALK LIKE A CELL BIOLOGIST
COLLAGEN – a major structural protein in the body, found in skin, bones and connective tissues. Type I collagen is the main component of bones
CRISPR – a genetic engineering tool that involves cutting and inserting genetic material in the DNA
EPITHELIAL CELLS – a specialised type of cell that lines the surface of organs, typically acting as a protective barrier
GENE THERAPY – modifying a person’s genes to treat or cure disease
IN VITRO – a process taking place in the lab (e.g. in a test tube); the opposite of in vivo (in a living organism)
OSTEOBLAST – a specialist type of cell that produces a bone matrix. Bone is not uniformly solid but consists of a flexible matrix (about 30%) and bound minerals (about 70%) that are intricately woven and endlessly remodelled by osteoblasts and osteoclasts. To read more about this, visit: https://en.m.wikipedia.org/wiki/Bone
OSTEOCLAST – a specialist type of cell that resorbs the old bone matrix
OSTEOGENESIS IMPERFECTA – also known as brittle bone disease, osteogenesis imperfecta is a rare genetic disease that starts in the womb and leads to deformities of the skeleton and breaking bones (fractures)
PLURIPOTENT – used to describe stem cells that can create all the different cell types present in the body, but are not totipotent (i.e. cannot create the placenta or umbilical cord)
STEM CELLS – unspecialised cells that are used as the ‘raw material’ to produce specialised cells
THERAPEUTIC – a therapy or medical treatment
“Our bones are extremely important, not just for supporting our body and protecting our internal organs, but also as a reservoir of minerals and other substances involved in metabolism,” says Dr Pascale V Guillot. “It is therefore very important to maintain bone health.” Pascale and her team are part of the Elizabeth Garrett Anderson Institute for Women’s Health at University College London in the UK. They are performing research involving some truly cutting-edge techniques: growing human bone tissue in the lab, developing stem cell-based therapeutics, reprogramming cells to pluripotency and editing the genetic makeup of stem cells to correct genetic defects.
Bone fragility affects many people for different reasons. Menopause, inactivity or immobility, and even lack of gravity (such as astronauts experience) can all weaken bones. Fragile bones can also be genetic: osteogenesis imperfecta, or brittle bone disease, is a rare inheritable disease that leads to fractures, short stature and skeletal deformities. Pascale and her team are focusing on this disease, but their findings will also help many people with bone fragility.
BONE BIOLOGY
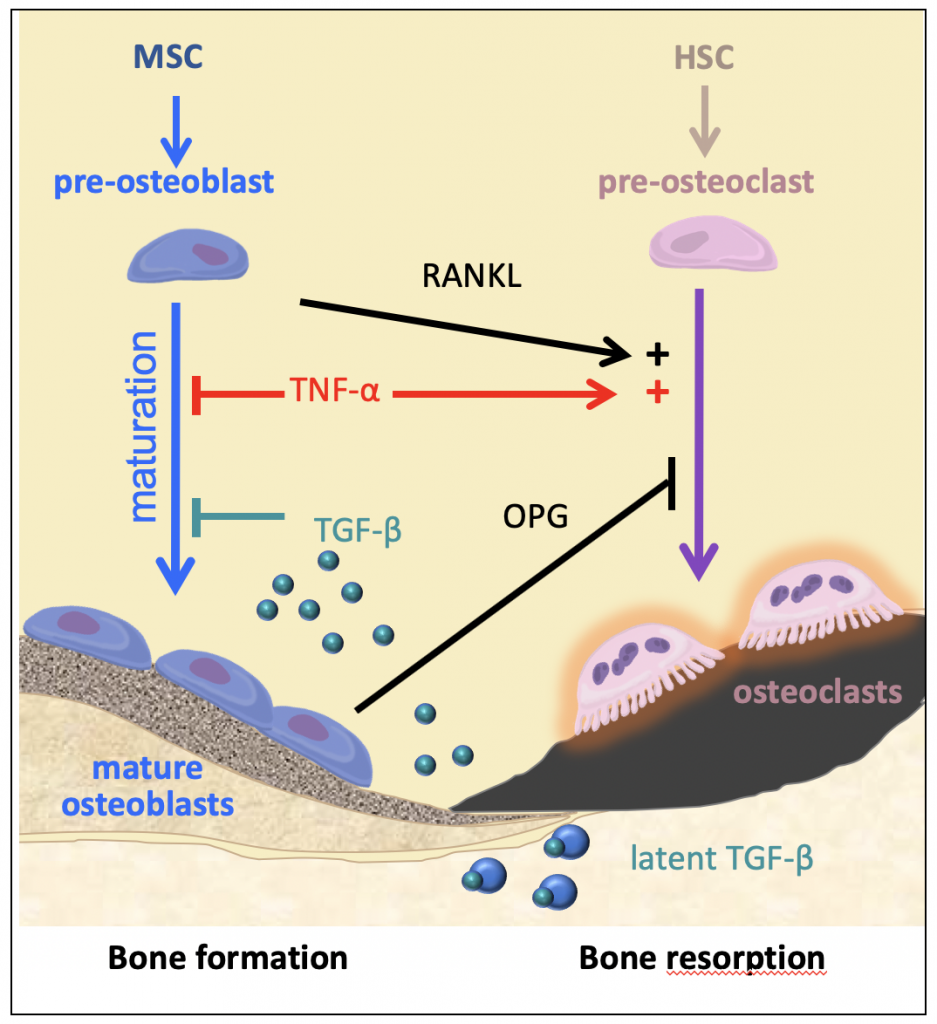
Bones may seem like relatively simple parts of the body, but there is plenty to them that does not initially meet the eye. “Bones are complicated structures, and are constantly remodelling,” says Pascale. “Cells called osteoblasts build new bone tissue, while cells called osteoclasts destroy old bone tissue – ideally at the same rate.” The osteoblasts produce fibres of a protein called type I collagen, which is a major building block within our bodies, contributing to skin, muscles and tendons, as well as bones. Once the osteoblasts have secreted the collagen, they then control the deposition of calcium and other minerals on these fibres, which add strength and structure to our skeleton.
Osteogenesis imperfecta is the result of errors (mutations) in genes that lead to insufficient or abnormal production of collagen fibres in bones. “We originally thought that the faulty fibres were directly responsible for weak bones, but it turns out there is more to it than that,” says Pascale. “Faulty fibres do lead to a weak bone structure – imagine a house made of cardboard bricks. However, these fibres also prevent osteoblasts from maturing properly, meaning they aren’t able to mineralise the fibres. So, as well as the house being made from cardboard, it also has fewer bricks than it should.” Added to this, reduced osteoblast activity also leads to increased osteoclast activity, meaning that bone destruction is happening faster than bone construction.
Now, Pascale’s team has an understanding of the pathway that leads from genetic mutations to bone fractures, they can move on to tackling osteogenesis imperfecta.
BUILDING BONES IN THE LAB
Pascale’s team is extracting epithelial cells found naturally in samples of people’s urine. Though these cells are already fully specialised, the team use a ground-breaking technique to return them to a pluripotent stem cell state. This cell rejuvenating technique, known
as cellular reprogramming to pluripotency (induced pluripotent stem cells, iPSCs), is revolutionising cell biology. Previously, nonpluripotent stem cells (called multipotent stem cells) were mainly isolated from various tissues of healthy donors and grown in the laboratory. However, as they grow, they also start to age and progressively lose their ability to specialise into bone-forming cells (osteoblasts) and repair tissues. Now, cell biologists can derive osteoblasts directly from iPSCs. “The iPSCs we create have the potential to become any type of specialist cell in the body,” says Pascale. “For our research, we cultivate them to become osteoblasts.”
Using these osteoblasts, Pascale’s team is working to build three-dimensional structures made up of human bone in vitro. “This technique means we don’t have to experiment using animals, and increases the physiological relevance of our work,” says Pascale. “However, we have several challenges to overcome, namely engineering a system where human cells can thrive and produce a bone matrix.” Bones are complex tissues requiring precise processes to build them correctly, so this is no easy task, but the team is making strong progress.
FROM EXPERIMENTS TO TREATMENTS
“Using urine from healthy patients and osteogenesis imperfecta patients, we’re able to build healthy bone structures and fragile bone structures,” says Pascale. “We can then test the effects of different treatments on the fragile bone structures, seeing if any stimulate the osteoblasts to mature properly and improve bone strength.” Excitingly, the team is also involved in a clinical trial that will determine the safety and efficacy of prenatal cell therapy for osteogenesis imperfecta. Since osteogenesis imperfecta is a genetic disease, there is another possible solution: editing the genes themselves to repair the genetic mutation.
“Using patients’ cells, we can correct the mutation using CRISPR, and then transplant these corrected cells back into the patients,” says Pascale. This technique has even greater potential than stem cell transplants, since the human body is much more likely to accept its own cells than those from a different person.
When will we see the team’s research in action? As Pascale explains, “The next step is to move from discovery to commercialisation, so these techniques can help individuals with osteogenesis imperfecta – and potentially people with other fragile bone conditions – across the world!”
Reference
https://doi.org/10.33424/FUTURUM144
COLLAGEN – a major structural protein in the body, found in skin, bones and connective tissues. Type I collagen is the main component of bones
CRISPR – a genetic engineering tool that involves cutting and inserting genetic material in the DNA
EPITHELIAL CELLS – a specialised type of cell that lines the surface of organs, typically acting as a protective barrier
GENE THERAPY – modifying a person’s genes to treat or cure disease
IN VITRO – a process taking place in the lab (e.g. in a test tube); the opposite of in vivo (in a living organism)
OSTEOBLAST – a specialist type of cell that produces a bone matrix. Bone is not uniformly solid but consists of a flexible matrix (about 30%) and bound minerals (about 70%) that are intricately woven and endlessly remodelled by osteoblasts and osteoclasts. To read more about this, visit: https://en.m.wikipedia.org/wiki/Bone
OSTEOCLAST – a specialist type of cell that resorbs the old bone matrix
OSTEOGENESIS IMPERFECTA – also known as brittle bone disease, osteogenesis imperfecta is a rare genetic disease that starts in the womb and leads to deformities of the skeleton and breaking bones (fractures)
PLURIPOTENT – used to describe stem cells that can create all the different cell types present in the body, but are not totipotent (i.e. cannot create the placenta or umbilical cord)
STEM CELLS – unspecialised cells that are used as the ‘raw material’ to produce specialised cells
THERAPEUTIC – a therapy or medical treatment
“Our bones are extremely important, not just for supporting our body and protecting our internal organs, but also as a reservoir of minerals and other substances involved in metabolism,” says Dr Pascale V Guillot. “It is therefore very important to maintain bone health.” Pascale and her team are part of the Elizabeth Garrett Anderson Institute for Women’s Health at University College London in the UK. They are performing research involving some truly cutting-edge techniques: growing human bone tissue in the lab, developing stem cell-based therapeutics, reprogramming cells to pluripotency and editing the genetic makeup of stem cells to correct genetic defects.
Bone fragility affects many people for different reasons. Menopause, inactivity or immobility, and even lack of gravity (such as astronauts experience) can all weaken bones. Fragile bones can also be genetic: osteogenesis imperfecta, or brittle bone disease, is a rare inheritable disease that leads to fractures, short stature and skeletal deformities. Pascale and her team are focusing on this disease, but their findings will also help many people with bone fragility.
BONE BIOLOGY
Bones may seem like relatively simple parts of the body, but there is plenty to them that does not initially meet the eye. “Bones are complicated structures, and are constantly remodelling,” says Pascale. “Cells called osteoblasts build new bone tissue, while cells called osteoclasts destroy old bone tissue – ideally at the same rate.” The osteoblasts produce fibres of a protein called type I collagen, which is a major building block within our bodies, contributing to skin, muscles and tendons, as well as bones. Once the osteoblasts have secreted the collagen, they then control the deposition of calcium and other minerals on these fibres, which add strength and structure to our skeleton.
Osteogenesis imperfecta is the result of errors (mutations) in genes that lead to insufficient or abnormal production of collagen fibres in bones. “We originally thought that the faulty fibres were directly responsible for weak bones, but it turns out there is more to it than that,” says Pascale. “Faulty fibres do lead to a weak bone structure – imagine a house made of cardboard bricks. However, these fibres also prevent osteoblasts from maturing properly, meaning they aren’t able to mineralise the fibres. So, as well as the house being made from cardboard, it also has fewer bricks than it should.” Added to this, reduced osteoblast activity also leads to increased osteoclast activity, meaning that bone destruction is happening faster than bone construction.
Now, Pascale’s team has an understanding of the pathway that leads from genetic mutations to bone fractures, they can move on to tackling osteogenesis imperfecta.
BUILDING BONES IN THE LAB
Pascale’s team is extracting epithelial cells found naturally in samples of people’s urine. Though these cells are already fully specialised, the team use a ground-breaking technique to return them to a pluripotent stem cell state. This cell rejuvenating technique, known
as cellular reprogramming to pluripotency (induced pluripotent stem cells, iPSCs), is revolutionising cell biology. Previously, nonpluripotent stem cells (called multipotent stem cells) were mainly isolated from various tissues of healthy donors and grown in the laboratory. However, as they grow, they also start to age and progressively lose their ability to specialise into bone-forming cells (osteoblasts) and repair tissues. Now, cell biologists can derive osteoblasts directly from iPSCs. “The iPSCs we create have the potential to become any type of specialist cell in the body,” says Pascale. “For our research, we cultivate them to become osteoblasts.”
Using these osteoblasts, Pascale’s team is working to build three-dimensional structures made up of human bone in vitro. “This technique means we don’t have to experiment using animals, and increases the physiological relevance of our work,” says Pascale. “However, we have several challenges to overcome, namely engineering a system where human cells can thrive and produce a bone matrix.” Bones are complex tissues requiring precise processes to build them correctly, so this is no easy task, but the team is making strong progress.
FROM EXPERIMENTS TO TREATMENTS
“Using urine from healthy patients and osteogenesis imperfecta patients, we’re able to build healthy bone structures and fragile bone structures,” says Pascale. “We can then test the effects of different treatments on the fragile bone structures, seeing if any stimulate the osteoblasts to mature properly and improve bone strength.” Excitingly, the team is also involved in a clinical trial that will determine the safety and efficacy of prenatal cell therapy for osteogenesis imperfecta. Since osteogenesis imperfecta is a genetic disease, there is another possible solution: editing the genes themselves to repair the genetic mutation.
“Using patients’ cells, we can correct the mutation using CRISPR, and then transplant these corrected cells back into the patients,” says Pascale. This technique has even greater potential than stem cell transplants, since the human body is much more likely to accept its own cells than those from a different person.
When will we see the team’s research in action? As Pascale explains, “The next step is to move from discovery to commercialisation, so these techniques can help individuals with osteogenesis imperfecta – and potentially people with other fragile bone conditions – across the world!”
 DR PASCALE V GUILLOT
DR PASCALE V GUILLOT
Associate Professor and Group Head
Elizabeth Garrett Anderson Institute for Women’s Health
University College London, UK
FIELD OF RESEARCH: Stem Cell Biology
RESEARCH PROJECT: Combining stem cell and gene editing techniques to develop the next generation of treatments for osteogenesis imperfecta.
FUNDER: UK Research and Innovation, Medical Research Council
 DR PASCALE V GUILLOT
DR PASCALE V GUILLOT
Associate Professor and Group Head
Elizabeth Garrett Anderson Institute for Women’s Health
University College London, UK
FIELD OF RESEARCH: Stem Cell Biology
RESEARCH PROJECT: Combining stem cell and gene editing techniques to develop the next generation of treatments for osteogenesis imperfecta.
FUNDER: UK Research and Innovation, Medical Research Council
ABOUT CELL BIOLOGY
Cell biology covers the fundamental units of life: the cells that make up every living organism on the planet. Cell biologists study the structure and function of these cells and how they work together to form whole organs and organisms. Often, discoveries in cell biology have big implications for medicine, so cell biologists will regularly work alongside clinicians, pharmaceutical developers and medical professionals. Pascale explains more about her career.
WHAT DOES THE FUTURE HOLD FOR CELL BIOLOGY?
My team and I are very excited to work with stem cells to generate transformative therapeutic techniques. We are passionate about understanding how donor stem cells work to repair tissue – rather than forming healthy tissue themselves, it looks like they mainly contribute by releasing sacs of molecules that activate the host’s own repair system.
IS THERE A CELL BIOLOGIST YOU PARTICULARLY ADMIRE?
Marie Curie is an excellent role model: a mother, a wife and a pioneering scientist who won two Nobel prizes. She admitted juggling these was a challenge: “I have frequently been questioned, especially by women, how I could reconcile family life with a scientific career. Well, it has not been easy.” Yet, Marie Curie maintained the self-belief that led to her breakthroughs: “We must have perseverance and above all confidence in ourselves. We must believe that we are gifted for something and that this thing, at whatever cost, must be attained.”
WOULD YOU RECOMMEND A CAREER IN CELL BIOLOGY?
Absolutely. We can make wonderful discoveries, and often work hand-in-hand with clinicians to help develop new treatments and improve people’s quality of life, all while increasing our knowledge of the processes underlying how bodies work.
ARE THERE MANY CAREER OPPORTUNITIES FOR CELL BIOLOGISTS IN THE UK?
Yes, cell biology is an international career, including in the UK. We have prestigious universities where academic careers are very possible, as well as world-leading pharmaceutical companies that often take on young people.
ELIZABETH GARRETT ANDERSON: HER LEGACY CONTINUES

Elizabeth was born in 1836 at a time when women’s rights were largely limited. Despite vigorous opposition from the medical establishment, she passed her medical exams in 1865 and became the first female doctor to qualify. Elizabeth fought tirelessly for women to have high-quality healthcare and the right for women to practise medicine, and, aged 36, she founded the first hospital for women in Britain.
After her death in 1917, the hospital was renamed the Elizabeth Garrett Anderson Hospital. This hospital no longer exists but Elizabeth’s legacy continues with the Elizabeth Garrett Anderson Institute for Women’s Health at University College London.
https://www.ucl.ac.uk/womens-health/aboutinstitute/our-history
EXPLORE A CAREER IN CELL BIOLOGY
• The British Society for Cell Biology (BSCB) has a page dedicated to careers and courses: https://bscb.org/learning-resources/softcell-elearning/careers-and-courses/
BSCB also offers financial support for high calibre undergraduate students, who wish to gain research experience in cell biology during their summer holidays: https://bscb.org/competitions-awardsgrants/studentships/
• Many academic and research institutions engage with schools, including on the subject of cell biology. For instance, University College London (where Pascale works) regularly partners with The Sutton Trust to deliver summer schools and discovery days.
• According to PayScale, the average salary for a cell biologist in the UK is £33,320.
Pascale recommends taking mathematics, biology, physics and chemistry at school to prepare for a relevant undergraduate degree. Many undergraduate degrees can lead to a career in cell biology – examples include biology, chemistry, medicine, natural sciences and biochemistry.
HOW DID PASCALE BECOME A CELL BIOLOGIST?
YOU HAVE A BSC IN MATHEMATICS AND PHYSICS AND A PHD IN NEUROSCIENCE. HOW HAVE THESE DIVERSE SUBJECTS AIDED YOUR CAREER?
I feel mathematics and physics are essential in biology as we often make calculations and build models. Cell biology can be approached from many different angles – for instance, neuroscience involves the study of cells of the nervous system. Even though neuroscience involves different types of cells to the ones I work with now, all cell biology is centred on understanding how our organs work at the cellular level. This understanding can be applied in many different contexts.
WHAT INSPIRED YOU TOWARDS A SCIENTIFIC CAREER?
My inspiration came from very early contact with the scientific community through my parents. I have always thrived on challenges and working on the edge of discovery to pioneer new therapeutics.
WHAT SETBACKS HAVE YOU EXPERIENCED IN YOUR LIFE?
I used to compete in high-level taekwondo competitions. Though I never aimed to become a professional athlete, I have always believed a connection between body and mind is a great complement to intellectual work. However, taekwondo left me paralysed in one leg, and it took me ten years from the moment I was told I would never walk again to be able to walk without limping and eventually start a new path with yoga.
WHAT ARE YOUR HOPES FOR THE FUTURE?
I hope that we keep on discovering how to live longer lives that are healthier both physically and mentally.
PASCALE’S TOP TIPS
01 Never stop dreaming and persevering. Your goals are within reach.
02 Science is all about failure. Failure will ultimately make you succeed.
Write it in the comments box below and Pascale will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)












I have a spinal cord injury from an ependymoma tumor inside my spinal cord, there from birth. It started doing damage in my twenties (probably before). It hurt to eat. I became very thin and anorexic. I had blood transfusions. The tumor went undiagnosed until I was 40 years old. Now I have an incomplete paralysis from the removal of the tumor at N.Y.U. by Neurosurgeon Fred J. Epstein. He kept me walking but I had and have 24/7 extreme pain. Now my neck needs surgery, from where the tumor pushed up on the neck and fluid. The Doctor’s say I will not survive the surgery, if I do not strengthen my bones. They have given me 6 months to increase my bone density. The bone medicine that builds bones makes me very sick. I would like to try regeneration of my bones and stem cells to help me heal. The Doctor’s say I am too fragile. On the other hand, I need the surgery and will lose the function of my hands and arms without the surgery. The pain would also increase. Do you have any suggestions?
Thank you very much!!!