Can we engineer bacteria to regenerate tissues?
Dr Christopher Contag, a biomedical engineer and microbiologist at Michigan State University in the US, is creating engineered endosymbionts – bacterial cells that can be delivered into cells of other organisms where they can persist and control cellular functions. Since these engineered endosymbionts have the potential to guide the regeneration of our organs and tissues, Chris hopes his work will help to rebuild tissues in patients with damaged or diseased organs.
Talk like a biomedical engineer
Cytoplasm — the liquid in a cell
Endosymbiont — a symbiont that lives inside the cell of a different species
Eukaryote — an organism with cells containing a nucleus and other organelles, e.g., a plant or animal
Genome — the complete set of genetic material in an organism
Host cell — the cell in which an endosymbiont lives
Macrophage — an immune cell responsible for removing infectious agents from an organism and rebuilding tissues
Organelle — a structure with a lipid membrane within a eukaryotic cell, including mitochondria (where respiration occurs) and chloroplasts (where photosynthesis occurs)
Prokaryote — a relatively simple single-celled organism that does not contain a nucleus or organelles, e.g., a bacterium
Stem cell — a non-specialised cell that can differentiate into (become) a specialised cell
Symbiont — an organism that lives in symbiosis with another species
Symbiosis — a close biological relationship between organisms of different species
Tissue — a group of eukaryotic cells that work together to perform a specific function, e.g., liver tissue, heart tissue or lung tissue
If one of your organs fails or becomes damaged, the best option may be to replace it with an organ donated by someone else. However, while organ transplants are life-changing for many patients, they are also expensive, risky and in extremely high demand. At any one time in the US, about 100,000 people are on the waiting list for organ donations. If you need a new heart or lung, you will have to wait about four months, while the average waiting time for a donated kidney is five years.
“There is a need to develop new tools to repair or replace damaged organs and tissues,” says Dr Christopher Contag, a biomedical engineer and microbiologist at Michigan State University. “If this could be done inside the body, it would avoid some of the risks and costs of organ transplantation and remove the need for donated organs.” Such tools could be used broadly across many organ systems, for example, to repair damaged heart tissue after a heart attack or regrow neurons to reverse the effects of Parkinson’s disease. “We propose to create engineered endosymbionts that can reprogram cells to become stem cells that, with the flip of a genetic switch in the endosymbiont, can activate a pathway to turn that stem cell into a specific tissue cell, such as a heart cell, liver cell or neuron,” explains Chris. “We could then direct cells in the body to repair or replace damaged tissues, while controlling them from outside the body.” While this may sound like the work of science fiction, Chris believes it could become a reality that revolutionises medicine. He has established this new scientific field of endosymbiont engineering for human health in the hope of improving health outcomes for patients with damaged and diseased tissues.
What is an endosymbiont?
Symbiosis is a close biological relationship between two organisms of different species (known as symbionts). For example, the lichen you might see growing on trees or tombstones is not a single organism, but a symbiotic relationship between algae and fungi. The algae provide nutrients for both organisms through photosynthesis, while the fungi provide support and shelter.
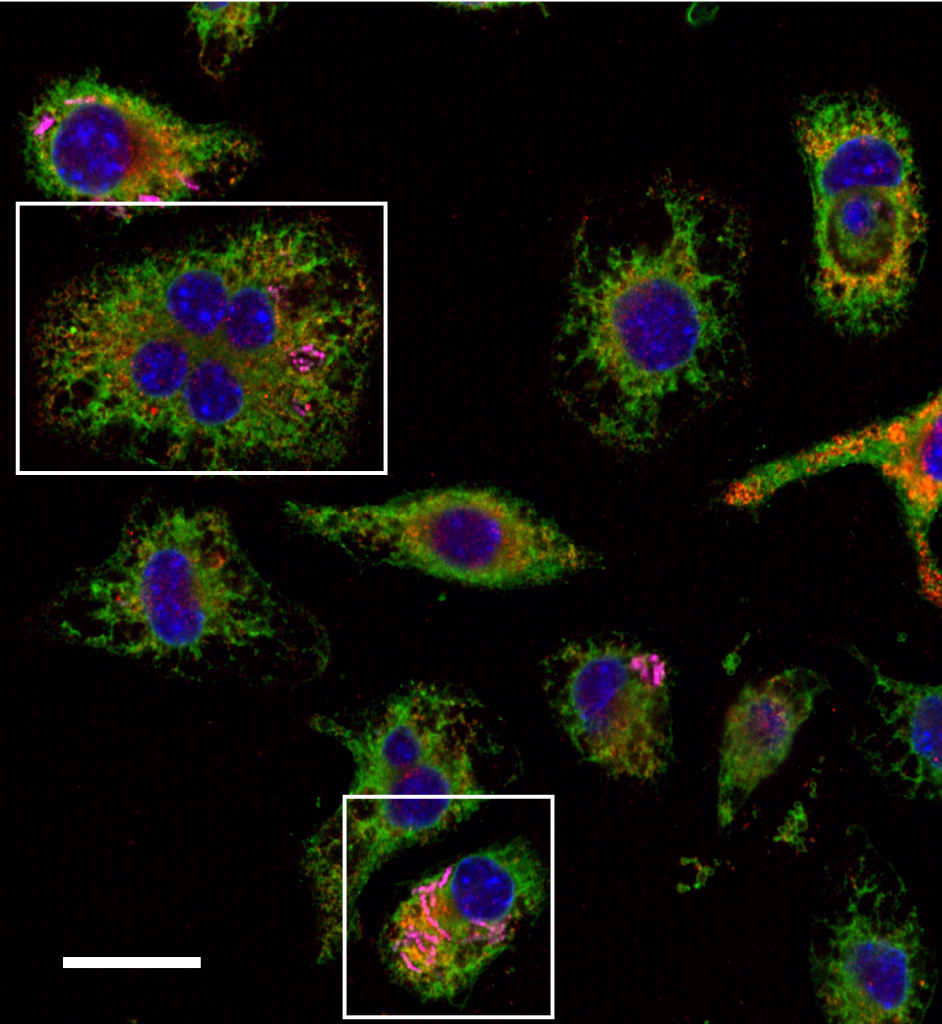
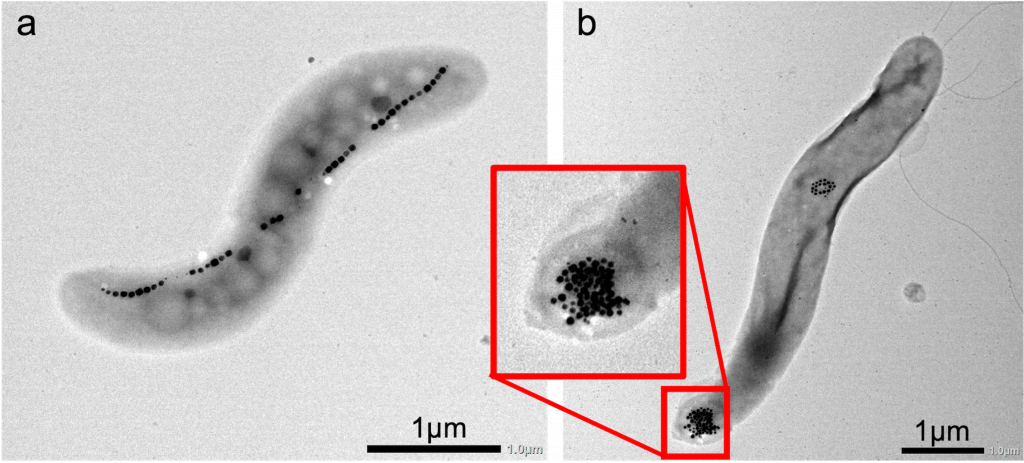
An endosymbiont is a symbiont that lives inside a cell of another species (known as the host cell). “Evolution used the process of endosymbiosis to create eukaryotes,” explains Chris. The endosymbiont theory states that eukaryotic cells evolved when one prokaryotic cell engulfed another, then the two cells co-evolved with one prokaryote existing as an endosymbiont within the other. Over about two billion years, the genomes and biologies of the two prokaryotic species became interdependent and were optimised to live harmoniously together. The endosymbionts evolved into organelles, such as mitochondria and chloroplasts, resulting in eukaryotic cells. “Engineered endosymbionts are bacterial cells that have been artificially engineered to create synthetic symbiotic relationships, so they exist as a cell living inside a cell of another species,” explains Chris.
How does Chris create and control engineered endosymbionts?
“We started the field of endosymbiont engineering with the idea that we could replicate the evolutionary process of endosymbiosis in the laboratory,” explains Chris. “We hope to create new endosymbiotic relationships, where the engineered endosymbionts are designed and built to control the biological processes of the cell in which it lives.” These engineered endosymbionts are bacterial cells that are usually free-living prokaryotes, but have been artificially modified so they can exist as synthetic organelles in the cytoplasm of mammalian host cells.
To create an engineered endosymbiont, Chris and his team genetically modify innocuous (harmless) bacteria of various species. They add genes encoding eukaryotic functions that interact with the host cell. The team will then reduce the endosymbiont’s genome to its minimal size and modify it so it is more compatible with the mammalian host cell. “We also build biological indicator lights, based on luminescing and fluorescing proteins, into either the host cell or the bacterial endosymbiont,” says Chris. “This lets us locate the endosymbionts in the body and monitor whether the engineered genes are ‘on’ or ‘off’. It also helps us determine whether the system is working, much like the warning lights in a car that indicate whether something is functioning or not.”
Reference
https://doi.org/10.33424/FUTURUM422
The overall goal is to link the biology of the bacterial and mammalian cells so scientists can control the function of the host cell by controlling the endosymbiont. It is important that the endosymbiont genes can be controlled in a way that does not interfere with the biochemical processes happening in the mammalian host cell. To achieve this, the team controls the endosymbiont genes using either sugars that mammalian cells do not use, heat produced by lasers, ultrasound or alternating magnetic fields.
Can we use these engineered endosymbionts for medical purposes?
While Chris and his colleagues have had many successes developing their engineered endosymbionts, it will be a while before they are used in medicine to direct the regrowth of hearts and kidneys. So far, the team has successfully used engineered endosymbionts to reprogram macrophages in the body to build new tissues, instead of fulfilling their alternate function of removing pathogens or dead cells. However, this has not been easy. Some of the main challenges faced by the team are encouraging the host cells to accept the engineered endosymbionts and encouraging the endosymbionts to better tolerate the host cells. “Mammalian cells have evolved many mechanisms to eliminate bacterial cells, even engineered ones, from their cytoplasm,” says Chris. “We need to overcome these mechanisms before we can create true symbiotic relationships between the host cell and engineered endosymbiont.”
This will be key to increasing the length of time that the endosymbionts persist in the host cells before they are rejected. At present, engineered endosymbionts can persist for a few days in a mammalian host cell, but it may take weeks or months to rebuild tissues. “We also want to introduce endosymbionts into a range of different cell types,” Chris says, “and we want to demonstrate that engineered endosymbionts can reprogram cells to become stem cells.”
This last step will be essential if engineered endosymbionts are to be used for tissue regeneration. Chris hopes to insert genes into the endosymbionts that reprogram mammalian host cells into stem cells. If these stem cells can be controlled by the endosymbionts, which, in turn, are being controlled from outside the body, then scientists could program cells inside the body to rebuild and repair damaged tissues, by instructing the stem cells to become whatever type of cell is necessary.
“Controlling tissue regeneration from outside the body still seems like science fiction to many people,” says Chris. This is both an achievement and a challenge when it comes to the team’s research. On the one hand, it shows just how innovative and cutting-edge these ideas are, highlighting how Chris is pushing the boundaries of scientific understanding and possibilities. On the other hand, some scientists remain sceptical about the realities of inserting bacteria into people to repair their organs. However, bacteriotherapy (which uses bacteria to kill tumours) has become standard treatment for bladder cancer and is now being investigated to treat a variety of other cancers. “We can build on these successes if we are methodical and transparent in our designs to ensure that the scientific community accepts this novel approach to guiding tissue regeneration,” says Chris.
With further advances in the field of endosymbiont engineering, these bacteria may one day be able to regrow damaged human tissues, eliminating the need for organ transplants.
 Dr Christopher Contag
Dr Christopher Contag
Institute for Quantitative Health Science and Engineering, Colleges of Engineering and Human Medicine, Michigan State University, USA
Fields of research: Biomedical Engineering, Endosymbiont Engineering, Medical Imaging, Microbiology
Research project: Developing engineered endosymbionts that can repair tissues in the body
Funders: US National Science Foundation (NSF), National Institutes of Health (NIH)
About biomedical engineering
Biomedical engineering lies at the intersection of biology and engineering, while also drawing on knowledge from medicine and chemistry. While some biomedical engineers create mechanical or electronic devices for medical use, such as pacemakers and prosthetic limbs, others, like Chris, apply engineering techniques to biological systems, for purposes such as gene therapy, gene editing and tissue engineering. “Biomedical engineering is a broad field that can impact everything from food production to space exploration,” says Chris. “In this field, your primary limitation is your ability, or inability, to imagine the future!”
The importance of futuristic thinking
When Chris realised that the challenges of organ donation and transplantation could be solved by regrowing tissues in the body, he began to investigate the possibility of engineering synthetic endosymbionts. In doing so, he established a new subfield of biomedical engineering. And, while some view his work as science fiction, Chris has proven that the concepts behind his ideas are theoretically and practically possible.
As technology and biological understanding advance, great progress can be expected in all fields of biomedical engineering. “In the field of endosymbiont engineering, I think we will develop bacteria that only contain the minimal number of genes needed for their growth, and we will generate libraries of genes that drive the development of different cells and tissues,” predicts Chris. “With this, we could customise the minimal genome bacteria by inserting other genes, so the bacteria perform different functions. For example, after a heart attack, a patient could be prescribed a minimal genome bacterium with heart generating genes that would guide the patient’s heart cells to regenerate.”
Progress in biomedical engineering relies on the next generation of scientists having a futuristic mindset and a willingness to pursue the unknown. As the chemist, Frank Westheimer, once said, “Progress is made by young scientists who carry out experiments that old scientists said wouldn’t work.” Chris acknowledges that some scientists call his work ‘science fiction’, in part due to a fear of the unknown. “Science fiction writers ask, ‘What if?’,” he says. “As scientists, we think of what might be possible and ask, ‘What’s the next step?’ We begin with the end in mind and direct our experiments toward this goal. However, innovations lie in the unexpected results, and therefore we must go into every design and experiment with our eyes wide open to appreciate the unexpected and then explain it.”
Pathway from school to biomedical engineering
- “All engineering fields require math,” says Chris. “So, get a solid education in math and quantitative sciences.” Study mathematics, biology, chemistry and physics and, if your school offers classes in engineering and computing, take them as well, since these subjects will also be useful.
- Some universities offer degrees in biomedical engineering, bioengineering or biological engineering. Related degrees in biochemistry, biology or other engineering fields (e.g., mechanical or electrical) could also lead to a career in biomedical engineering.
Explore careers in biomedical engineering
• “The Biomedical Engineering Society (BMES; www.bmes.org) and the Engineering in Medicine and Biology Society (EMBS; www.embs.org) are outstanding organisations to get involved with,” says Chris. Both societies have a wealth of resources, including podcasts and informative videos on topics such as ‘Is biomedical engineering right for you?’ and ‘Career paths in biomedical engineering’.
• The US Bureau of Labour Statistics has detailed information about careers in bioengineering and biomedical engineering, including what the roles involve, the skills and qualifications you will need and the salary you can expect: www.bls.gov/ooh/architecture-and-engineering/biomedical-engineers.htm
• In this video from the American Society of Mechanical Engineers, bioengineers discuss what work in the field involves: www.asme.org/topics-resources/content/video-bioengineering
Meet Chris
As far back as I can remember, I wanted to be a scientist. I was always fascinated by the processes of biology – while my friends were building model cars, I was building models of the human eye, ear and other organ systems! I saw the interconnections of biological systems while picking edible plants with my dad and spending days wandering and camping in the woods.
My father and grandfather were veterinarians and, while growing up, I spent many hours in their lab. My dad inspired curiosity in me as we grew bacteria, hatched turtle eggs, did chemistry experiments, blew glass bottles and treated sick animals that I found in the woods, from dogs and cats to squirrels, snakes and a mountain lion (though I didn’t bring the mountain lion back to the clinic!).
When I was a kid, there was a science fiction TV show called ‘The Time Tunnel’, about scientists who build a time tunnel and adventurers who travel through it. My brother wanted to be one of the heroic time travellers, but I wanted to be the chief scientist and figure out how the scientists could save the day. I still believe that scientists will save the day by developing innovative new technologies for addressing climate change and improving human health.
I was extremely lucky to have teachers who curated curiosity in their students. When I was 10, we were learning about eyes by looking at drawings in books. I didn’t think this was sufficient, so I asked my teacher if I could bring an eye to school to dissect in class. I don’t think she realised I was serious, because she just said, “Sure”. So later that week, when I helped my dad deliver a still-born calf, we removed its eye and the next day I took the eye, a dissection tray and the proper tools to school and offered them to my teacher. I still remember the expression on her face as she stepped back, and said, “Why don’t you teach today?” So, I led the class through the dissection of the calf eye. It wasn’t until many years later that I realised this might have seemed strange! To me, I was just bringing my everyday life into the classroom.
I never get tired of watching biology in action – from the molecular and cellular level while working in the lab, to the organismal and ecosystem level outside in nature. Whether in the lab, mountains or oceans, there are so many aspects of biology to observe and wonder about.
Chris’ top tips
1. Instead of ‘thinking outside the box’, just ‘think outside, no box required’. It is human nature to categorise and catalogue things, but every time we box something in, we eliminate opportunities.
2. Follow your passion, not the career with the highest salary or most manageable work week. To be happy, make your work your hobby and your hobby your work, by integrating your life and work so that what you accomplish in each area exceeds your and others’ expectations.
3. Never let your mind grow old – keep the youthful wonder alive. I like to think of myself as a young scientific mind in an old human body! I never want to be a scientist who says ‘no’ to trying something new.
Do you have a question for Chris?
Write it in the comments box below and Chris will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)