Can we vaccinate against the viruses hiding in our cells?
Many of us have viruses lying dormant within our cells. While our immune system controls these to keep us healthy, problems arise if our immune system becomes weakened. Dr Matthew Reeves is a molecular virologist at University College London, in the UK. He is developing a vaccine against human cytomegalovirus, a virus that infects 70% of the world’s population and can have devastating consequences for unborn babies and organ transplant patients
TALK LIKE A VIROLOGIST
ADAPTIVE IMMUNITY – an immune response that is directed against a specific virus
ANTIBODIES – proteins in the immune system which can recognise specific viral proteins to combat disease
HOST – a cell or organism within which a virus replicates itself
IMMUNE SYSTEM – our body’s defence system against infection
VIRUS – an infectious agent that causes disease by replicating within the cells of an organism
VIRUS LATENCY – the ability of a virus to exist indefinitely within its host in a dormant state
“It is estimated that over 70% of the world’s population is infected with human cytomegalovirus (HCMV),” says Dr Matthew Reeves, a molecular virologist at University College London. In some countries, prevalence is approaching 100%, meaning that almost every individual is infected. Yet most people will carry HCMV throughout their lives without ever noticing.
HCMV is a herpes virus, which usually causes mild symptoms that feel like a bad cold. It is one of nine herpes viruses that infect humans, with others causing chickenpox, glandular fever and cold sores.
WHAT HAPPENS WHEN A VIRUS ENTERS OUR BODY?
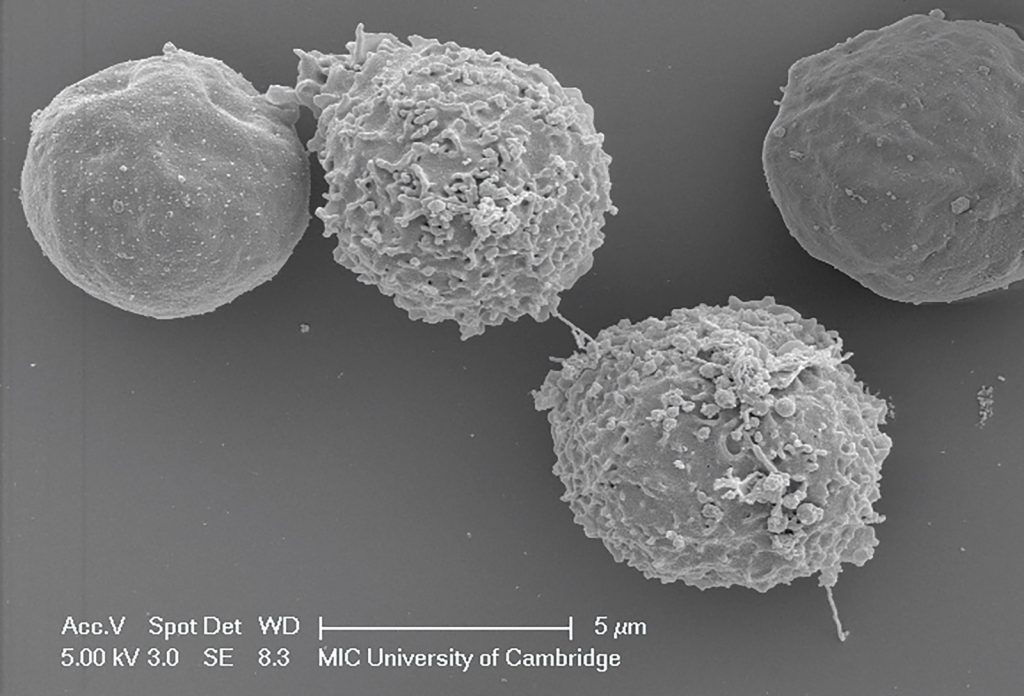
“Viruses are parasitic,” explains Matt. “They need cells, and all the wonderful things in them, to make copies of themselves.” Each virus particle contains the genetic instructions needed to replicate itself. Once the virus has replicated within a cell, the new copies infect other cells where they then replicate themselves. In this way, the infection spreads throughout the body, and hopefully (from the virus’s point of view) into other individuals. “The primary success criterion for any virus is transmission,” says Matt. “If a virus can infect a host, make copies, and then infect a new host, then it is successful.”
If a virus enters our body, our immune system leaps into action to defend us. “The adaptive immune response targets viruses in two ways,” explains Matt. “B cells make antibodies that can neutralise viruses to stop them from infecting cells, while T cells can recognise cells that have been infected with a virus and can destroy the cell and therefore the virus.”
VIRUS LATENCY
Sometimes, the immune system is successful in preventing a virus from replicating, but not in eliminating all the viral particles from the body. A virus remaining in the body in this state is said to be latent. “A latent virus can still deliver its genetic material to the host cell. Once there, the material is silenced but not eliminated,” Matt explains. “Importantly, the silencing is reversible – this genetic material can become active again and resume making new viruses.”
But even when a latent virus is reactivated, the infection doesn’t usually progress far before the immune system brings it under control. “Problems arise when your immune system is not working as well as normal,” says Matt. “A weakened immune system may allow the virus to escape, replicate extensively and ultimately cause disease.”
Once a person is infected with a herpes virus, they are infected for life, as herpes viruses have evolved to evade immune detection, allowing them to latently reside within us. As the latent virus does not cause harm, this is not a major problem, although it means the immune system must constantly work to control the virus. If a person has latent HCMV, more than 20% of their T cells may be dedicated to controlling just this one virus!
But if someone’s immune system becomes weakened, then they will no longer be able to supress latent viruses. This is why some people suffer from repeated bouts of glandular fever each time they become overly tired and run down, why people develop cold sores if they become stressed, and why adults can develop shingles decades after they had chickenpox as a child. In each case, the latent herpes virus is reactivating due to a weakening of the immune system.
GROUPS AT RISK FROM SERIOUS HCMV
While HCMV only causes cold-like symptoms in most people, it is serious in those without a functioning immune system. If a pregnant woman becomes infected with HCMV, her immune system will fight the virus, but the virus can be passed to the foetus (which has no immune system) across the placenta. This can result in blindness, deafness or developmental issues when the baby is born.
Organ transplant patients are also at risk of serious harm from HCMV. When a patient receives an organ transplant, there is a strong chance their immune system will attack the donated organ because it sees it as ‘foreign’ in the same way it recognises infections. Before the transplant takes place, doctors must therefore give the patient drugs to temporarily suppress their immune system. But this then provides any latent viruses with the opportunity to flourish.
“A good analogy of this medical dilemma is of a dog (HCMV) straining on a leash (being controlled by the immune system),” says Matt. “If we let go of the leash (immune suppression), the dog can run wild (HCMV replication and disease).” Doctors must balance the patient’s immune system by ensuring that it is not strong enough to reject the new organ but is strong enough to suppress any latent viruses.
DEVELOPING AN HCMV VACCINE
Matt and his team are investigating how the virus avoids immune detection with the aim of developing a vaccine to protect those most at risk of HCMV. If transplant patients could be vaccinated against HCMV, this would reduce the risks associated with immune suppression during and after the operation. Vaccinating women of child-bearing age could protect unborn babies from contracting HCMV in the womb. And vaccinating children could stop them spreading the virus. “Children are prodigious shedders of HCMV in urine and saliva,” says Matt. “As such, we often acquire the virus in childhood or through contact with children.”
Developing a vaccine for HCMV presents challenges, as much of the world’s population is already infected with the virus. This means the conventional approach of training the immune system before it encounters the virus may not work unless we can ‘re-educate’ the immune system effectively.
Reference
https://doi.org/10.33424/FUTURUM203
ADAPTIVE IMMUNITY – an immune response that is directed against a specific virus
ANTIBODIES – proteins in the immune system which can recognise specific viral proteins to combat disease
HOST – a cell or organism within which a virus replicates itself
IMMUNE SYSTEM – our body’s defence system against infection
VIRUS – an infectious agent that causes disease by replicating within the cells of an organism
VIRUS LATENCY – the ability of a virus to exist indefinitely within its host in a dormant state
“It is estimated that over 70% of the world’s population is infected with human cytomegalovirus (HCMV),” says Dr Matthew Reeves, a molecular virologist at University College London. In some countries, prevalence is approaching 100%, meaning that almost every individual is infected. Yet most people will carry HCMV throughout their lives without ever noticing.
HCMV is a herpes virus, which usually causes mild symptoms that feel like a bad cold. It is one of nine herpes viruses that infect humans, with others causing chickenpox, glandular fever and cold sores.
WHAT HAPPENS WHEN A VIRUS ENTERS OUR BODY?
“Viruses are parasitic,” explains Matt. “They need cells, and all the wonderful things in them, to make copies of themselves.” Each virus particle contains the genetic instructions needed to replicate itself. Once the virus has replicated within a cell, the new copies infect other cells where they then replicate themselves. In this way, the infection spreads throughout the body, and hopefully (from the virus’s point of view) into other individuals. “The primary success criterion for any virus is transmission,” says Matt. “If a virus can infect a host, make copies, and then infect a new host, then it is successful.”
If a virus enters our body, our immune system leaps into action to defend us. “The adaptive immune response targets viruses in two ways,” explains Matt. “B cells make antibodies that can neutralise viruses to stop them from infecting cells, while T cells can recognise cells that have been infected with a virus and can destroy the cell and therefore the virus.”
VIRUS LATENCY
Sometimes, the immune system is successful in preventing a virus from replicating, but not in eliminating all the viral particles from the body. A virus remaining in the body in this state is said to be latent. “A latent virus can still deliver its genetic material to the host cell. Once there, the material is silenced but not eliminated,” Matt explains. “Importantly, the silencing is reversible – this genetic material can become active again and resume making new viruses.”
But even when a latent virus is reactivated, the infection doesn’t usually progress far before the immune system brings it under control. “Problems arise when your immune system is not working as well as normal,” says Matt. “A weakened immune system may allow the virus to escape, replicate extensively and ultimately cause disease.”
Once a person is infected with a herpes virus, they are infected for life, as herpes viruses have evolved to evade immune detection, allowing them to latently reside within us. As the latent virus does not cause harm, this is not a major problem, although it means the immune system must constantly work to control the virus. If a person has latent HCMV, more than 20% of their T cells may be dedicated to controlling just this one virus!
But if someone’s immune system becomes weakened, then they will no longer be able to supress latent viruses. This is why some people suffer from repeated bouts of glandular fever each time they become overly tired and run down, why people develop cold sores if they become stressed, and why adults can develop shingles decades after they had chickenpox as a child. In each case, the latent herpes virus is reactivating due to a weakening of the immune system.
GROUPS AT RISK FROM SERIOUS HCMV
While HCMV only causes cold-like symptoms in most people, it is serious in those without a functioning immune system. If a pregnant woman becomes infected with HCMV, her immune system will fight the virus, but the virus can be passed to the foetus (which has no immune system) across the placenta. This can result in blindness, deafness or developmental issues when the baby is born.
Organ transplant patients are also at risk of serious harm from HCMV. When a patient receives an organ transplant, there is a strong chance their immune system will attack the donated organ because it sees it as ‘foreign’ in the same way it recognises infections. Before the transplant takes place, doctors must therefore give the patient drugs to temporarily suppress their immune system. But this then provides any latent viruses with the opportunity to flourish.
“A good analogy of this medical dilemma is of a dog (HCMV) straining on a leash (being controlled by the immune system),” says Matt. “If we let go of the leash (immune suppression), the dog can run wild (HCMV replication and disease).” Doctors must balance the patient’s immune system by ensuring that it is not strong enough to reject the new organ but is strong enough to suppress any latent viruses.
DEVELOPING AN HCMV VACCINE
Matt and his team are investigating how the virus avoids immune detection with the aim of developing a vaccine to protect those most at risk of HCMV. If transplant patients could be vaccinated against HCMV, this would reduce the risks associated with immune suppression during and after the operation. Vaccinating women of child-bearing age could protect unborn babies from contracting HCMV in the womb. And vaccinating children could stop them spreading the virus. “Children are prodigious shedders of HCMV in urine and saliva,” says Matt. “As such, we often acquire the virus in childhood or through contact with children.”
Developing a vaccine for HCMV presents challenges, as much of the world’s population is already infected with the virus. This means the conventional approach of training the immune system before it encounters the virus may not work unless we can ‘re-educate’ the immune system effectively.
Additionally, HCMV has evolved to exist, and even to thrive, in the face of immune responses. To avoid antibodies, HCMV limits the amount of time it spends outside our cells, and it expresses proteins to prevent infected cells alerting T cells to the presence of infection. So, Matt and his team are trying a new approach, to modify the way in which the immune system operates. “We are searching for the Achilles heel of the virus,” says Matt. “We want to find an immune response that the virus cannot evade, which we can enhance via use of a vaccine.”
Clinical trials have been conducted by other research groups attempting to develop an HCMV vaccine. While no suitable vaccine has yet been produced, some have been partially effective. By studying data from past trials, Matt is trying to distinguish which aspects within vaccines produced good immune responses in patients and which did not. “With this knowledge, we aim to devise strategies to enhance the good responses whilst limiting the less beneficial ones,” he says.
The team has found a potential Achilles heel of the HCMV virus, a vaccine-specific response that controls the virus. Next, they hope to develop a vaccine that takes advantage of this, enabling vaccinated people to make this immune response more effectively.
If you want to combine cutting-edge science with real-world impact, a career in virology could be for you!
 DR MATTHEW REEVES
DR MATTHEW REEVES
Associate Professor of Molecular Virology, Institute of Immunity and Transplantation, University College London, UK
FIELD OF RESEARCH: Molecular Virology
RESEARCH PROJECT: Developing a vaccine against human cytomegalovirus
FUNDER: Medical Research Council, Wellcome Trust, Rosetrees Trust, NIHR, Royal Free Charity (The contents are solely the responsibility of the authors and do not necessarily reflect the views of the Funding Agencies above.)
 DR MATTHEW REEVES
DR MATTHEW REEVES
Associate Professor of Molecular Virology, Institute of Immunity and Transplantation, University College London, UK
FIELD OF RESEARCH: Molecular Virology
RESEARCH PROJECT: Developing a vaccine against human cytomegalovirus
FUNDER: Medical Research Council, Wellcome Trust, Rosetrees Trust, NIHR, Royal Free Charity (The contents are solely the responsibility of the authors and do not necessarily reflect the views of the Funding Agencies above.)
ABOUT VIROLOGY
Virology is the study of viruses and the diseases they cause. But virology can also help answer other biological questions. Matt’s primary interest lies in understanding how our cells work and what goes wrong in them to cause disease, and he uses virology as a means to investigate this.
“If you want to understand how something works then ask an expert,” he says. “In the case of human cells, the viruses are the experts! They have been evolving to use our cells to their own advantage for millions of years.” This is why studying viruses is the best method we have for understanding our own cells. The first oncogene (a gene that causes cancer) was found in a virus.
And in this long relationship between humans and viruses, it’s not only the viruses that have been evolving. “A lot of our own DNA is the result of infection with a family of viruses that inserted their genome into our DNA earlier in the human story,” Matt explains. “For instance, the protein that is crucial for the formation of the placenta (syncitin) first came from a virus that infected our ancestors many years ago!”
WHAT IS A TYPICAL DAY FOR A MOLECULAR VIROLOGIST?
“There isn’t a typical day!” says Matt. “I think that is the fun of it!” Matt spends some days in the lab doing experiments, some days going to meetings to talk about his research, and some days reading about the research conducted by other scientists around the world. This means that he is always learning new things about viruses, diseases and cells. An important part of his job is teaching the next generation of scientists in the lecture theatre and laboratory.
HOW CAN VIRUSES INFECT NEW SPECIES?
“The process of co-evolution between a virus and its host means viruses end up being highly adapted to growing in a particular species,” explains Matt. “They need to be able to recognise specific host proteins in order to infect and replicate, whilst also avoiding recognition by other host proteins that would stop them replicating.”
It’s a bit like navigating a new and stressful social event! Being in an unfamiliar social environment can be difficult when you lack the cues that let you know what is expected of you and how you should behave. Similarly, when a virus finds itself within the cells of an unfamiliar species, it is less effective at recognising which proteins it needs and which it must avoid.
But sometimes, a virus can overcome this barrier and infect a new species. “When a virus is successful in infecting a new host species, it is usually as a result of a mutation which makes it better able to infect and grow in the new host. Scientists call this a zoonotic event,” Matt explains. The viruses that cause COVID-19, Ebola and AIDS all began in animals, but mutations enabled them to infect humans. And as we encroach on greater portions of natural habitat and therefore come into closer contact with wild species, opportunities for viruses to attempt the jump into humans will increase.
EXPLORE A CAREER IN VIROLOGY
• The ever-present threat of viruses means that virologists are in demand in many places. You may find yourself working on a hospital ward with patients, in a research lab studying viruses, in a biotechnology lab developing vaccines, or advising the government during a viral outbreak.
• It can be valuable to obtain experience in a research lab while still at school. Matt recommends the schemes run by the Nuffield Foundation (www.nuffieldfoundation.org/students-teachers/nuffield-research-placements) and In2Science (www.in2scienceuk.org/about), both of which enable school students to undertake science research placements.
• The Royal College of Pathologists provides information about how to become a virologist: www.rcpath.org/discover-pathology/careers-in-pathology/careers-in-medicine/become-a-virologist.html
• The Microbiology Society (www.microbiologysociety.org/members-outreach-resources.html) and the British Society for Immunology (www.immunology.org/public-information/immunology-related-activities-and-resources) both have resources and activities for aspiring virologists.
• Study maths and science subjects at school. Biology and chemistry A-levels are likely to be requirements for studying a virology-related degree.
• Matt also recommends gaining computing skills, as these will be essential for handling the large volumes of data generated in all fields of science.
• Many universities will offer undergraduate degrees in virology and/or immunology, or postgraduate degrees in infection and immunity. Other degrees, such as biochemistry or biomedical science, could also lead to a career in virology. You don’t have to start out as a virologist. Matt trained as a molecular biologist and specialised in virology when he began his PhD.
HOW DID MATT BECOME A VIROLOGIST?
WHAT INSPIRED YOU TO BECOME A SCIENTIST?
I was interested in detective stories as a child – the Sherlock Holmes stories were my favourites! I also enjoyed problem solving, so a career in science seemed like a natural fit. Another reason for wanting to become a scientist was that I liked the noble idea of doing something for the ‘common good’.
WHAT DO YOU MOST ENJOY ABOUT YOUR JOB?
I always love the thrill of finding new knowledge. When you make a scientific discovery, you are the first person in the world to know it! However, the part of my job that gives me the greatest pleasure now is seeing people I have trained in my lab go onto the next steps in their career and be successful.
WHAT ARE YOUR PROUDEST CAREER ACHIEVEMENTS?
My two proudest achievements came when I obtained my PhD from Cambridge, and when I won the Medical Research Council Fellowship which allowed me to start my own lab studying HCMV.
WHAT ARE YOUR AMBITIONS FOR THE FUTURE?
I hope to identify a vaccine against HCMV – though this could put me out of a job!
WHAT DO YOU ENJOY OUTSIDE WORK?
Sport is my major interest – I suffer supporting Sheffield United, my hometown club.
MATT’S TOP TIPS
01 Remember that science is simply about finding stuff out and then telling people about it!
02 Never forget that the most important question is “Why?” Always ask, “Why does that happen?” Then the next question is “How? How does this happen?”
03 Learn not to be disheartened by rejection! Unfortunately, all scientists suffer rejection of papers they wanted to publish or grants they wanted to have funded. It is important to bounce back from this.
Do you have a question for Matt?
Write it in the comments box below and Matt will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)