Combining medicine and research to treat children with tuberculosis
Dr James Seddon is a clinician scientist, working both as a medical doctor at St Mary’s Hospital and as a researcher at Imperial College London in the UK and Stellenbosch University in South Africa. He is conducting a clinical trial to test the effectiveness of a new drug at preventing children who have been exposed to multidrug-resistant tuberculosis from developing the disease. He also hopes to develop a test to identify children at greater risk of developing tuberculosis. James’s research could dramatically reduce the number of children who die from tuberculosis each year
TALK LIKE A CLINICIAN SCIENTIST
BACTERIA – single-celled microorganisms, some of which affect humans in beneficial or harmful ways
TUBERCULOSIS (TB) – a potentially-fatal bacterial infection that mainly affects the lungs and usually causes coughing, fever and weight loss
MULTIDRUG-RESISTANT TUBERCULOSIS (MDR-TB) – a form of TB where the bacteria are resistant to the best anti-TB drugs
PAEDIATRICIAN – a medical doctor who diagnoses and treats health conditions in children
CLINICAL TRIAL – a type of scientific study used to test the effectiveness of a medical treatment
PLACEBO – a non-active treatment given to participants in clinical trials
In 2020, 1.1 million children worldwide were estimated to develop tuberculosis (TB), a potentially fatal disease that primarily affects the lungs and usually causes coughing, fever and weight loss. TB is caused by the bacteria Mycobacterium tuberculosis, and when someone with TB coughs, these bacteria are released into the air. If someone else breathes them in, these bacteria enter that person’s lungs, resulting in TB infection. Many people with TB infection never become unwell, as their immune system controls the bacteria in the body, but sometimes the number of bacteria increases, at which point the person develops TB disease and becomes unwell.
The good news is that doctors can test for TB infection before the onset of TB disease, enabling patients to be treated with drugs to ensure they do not progress to disease. However, some forms of TB bacteria have evolved to become resistant to the standard anti-TB drugs, resulting in multidrug-resistant (MDR) TB. If someone is infected with MDR-TB, normal treatments are ineffective, and doctors cannot prevent them developing the disease.
Dr James Seddon is a clinician scientist working as both a paediatrician and researcher, who divides his time between St Mary’s Hospital and Imperial College London in the UK, and Stellenbosch University in South Africa. James is conducting research into childhood TB in South Africa, to establish whether a new drug can prevent children who have been exposed to MDR-TB from developing TB disease. He also hopes to develop a gene expression test to identify children at higher risk of developing the disease, so treatments can be targeted more efficiently.
WHAT HAPPENS DURING A CLINICAL TRIAL?
James and his colleagues are running a clinical trial to test whether a new drug, levofloxacin, is safe and effective at preventing TB disease in children who have been exposed to MDR-TB. Normally, when someone is diagnosed with TB, the children in their household are given anti-TB drugs to prevent future TB. But if the person with TB is diagnosed with MDR-TB, then the normal drugs that are given to prevent future TB are ineffective and so no treatment can be given to children in the household.
James’s team contacts adults who have been diagnosed with MRD-TB. If they live with any children under the age of five, they are asked whether they would like the child to join the clinical trial. To determine whether levofloxacin prevents children exposed to MDR-TB infection developing TB disease, James must assess how many children progress to the disease when taking the drug, compared to how many progress to the disease without treatment.
Each child is randomly assigned to either receive levofloxacin tablets or a placebo (a dummy pill that doesn’t contain any treatment). “Neither the research team nor the families know which children are receiving levofloxacin treatment and which are receiving the placebo,” explains James. “This is important, because if people knew which medicine the child was receiving, it may influence how they are treated which in turn may affect the study results.” This is called a ‘double-blind’ clinical trial.
The children take the tablets for six months, during which time they are examined by the medical team every few weeks. The medical team continues to regularly assess every child for a year after they finish their treatment. Any children who develop TB disease will be diagnosed early and will receive the best possible treatment. “We hope that even the children receiving the placebo will be better off than children exposed to MDR-TB who are not in the trial,” says James.
WHAT RESULTS DOES JAMES EXPECT TO SEE?
“We expect fewer of the children who are given levofloxacin to progress to TB disease, compared to the children given the placebo,” says James. If this is the case, and assuming there are no adverse side effects of the drug, then this will be good evidence that levofloxacin is safe and effective at preventing TB disease in children exposed to MDR-TB. It will then be the responsibility of health policy makers to introduce this new drug to global health policies. “We would hope that if we find that levofloxacin reduces risk of TB disease progression and is safe, then the World Health Organization will recommend that, in future, all children who have been exposed to MDR-TB should be given levofloxacin,” James says.
HOW CAN A CHILD’S TB RISK BE DETERMINED?
Throughout the course of the trial, blood samples are taken from every child at regular intervals and tested to ensure the drug is safe. James and his colleagues are also analysing these blood samples to determine which genes are activated in each child at each point in time. By comparing the activated genes in children who progress to TB disease with the activated genes in children who remain well, James hopes to identify which activated genes can tell these two groups of children apart. A clinical test could then be developed so that when a child is exposed to TB, their risk of developing TB disease can be determined by testing for these few key activated genes.
This gene expression test would involve taking a small blood sample from the child by pricking their finger and analysing this blood in a machine that identifies the key activated genes within about half an hour. Children most at risk of developing TB disease could then be given appropriate treatment. This would enable countries with high rates of TB to prioritise their often-limited medical resources for children who most need them.
“If this kind of test were widely available, it could dramatically increase the number of children who are appropriately given treatment for TB infection and reduce the number of children who develop TB and who die from the disease each year,” says James.
 DR JAMES SEDDON
DR JAMES SEDDON
Consultant, Department of Paediatric Infectious Diseases, St Mary’s Hospital, London, UK
Reader in Global Child Health, Department of Infectious Diseases, Imperial College London, UK
Associate Professor, Desmond Tutu TB Centre, Stellenbosch University, South Africa
FIELD OF RESEARCH: Childhood Tuberculosis
RESEARCH PROJECT: Conducting a clinical trial to determine the effectiveness of levofloxacin at preventing TB disease in children and developing a gene expression test to identify children at risk of developing TB
FUNDER: Medical Research Council (MRC)
ABOUT CLINICIAN SCIENTISTS
A clinician scientist works as both a medical doctor treating patients and as a researcher in their specialist field. James is a paediatrician who looks after children with infectious diseases while also conducting research to find new tests and treatments for those diseases. He feels these roles complement each other well.
“The clinical work keeps me on my toes, making me aware of the most important questions that need answering to improve the care of children,” he says. “The research is very satisfying as it allows me to answer some of those questions, by changing the way we understand, diagnose and treat diseases.” But this dual role can also be challenging. “By doing two separate jobs, you feel that you are never giving either of them enough of your attention.”
WHAT DOES A CLINICIAN SCIENTIST’S DAY LOOK LIKE?
On clinical days, James visits his patients and works with health staff to decide what tests and treatments each child might need. He also advises other paediatricians about infectious diseases. He is often on-call overnight, ready to go into the hospital if he needs to attend to his patients.
On research days, James mainly does analysis and writing at his computer and sometimes sees children clinically who are taking part in his studies. Research projects can take years from start to finish, and they involve multiple stages: developing ideas and project proposals, applying for funding and ethics approval, collecting and analysing data, and finally, getting the research published. James always has lots of projects on the go, all at different stages. As research is a collaborative effort, this means he attends lots of meetings with his colleagues to discuss the progress of projects.
WHAT PERSONAL QUALITIES SHOULD CLINICIAN SCIENTISTS HAVE?
James says that anyone pursuing a career as a clinician scientist should be open minded and continually asking questions about the world, like ‘how can this be improved?’ or ‘how can we understand this better?’ Being organised and able to multitask are also useful skills. Clinical work and research are both team activities so it is vital that you can work well with other people.
EXPLORE A CAREER AS A CLINICIAN SCIENTIST
• It is important that clinician scientists are interested both in treating patients and in scientific research. The following articles written by clinician scientists highlight the rewards and challenges of the job:
– www.ncbi.nlm.nih.gov/pmc/articles/PMC4485885
– journals.biologists.com/dmm/article/3/3-4/125/2335/Interested-in-a-career-as-a-clinician-scientist
• University outreach programmes can help you learn more about both aspects of this career. Imperial College London offers activities for young people to learn about science (www.imperial.ac.uk/be-inspired/schools-outreach) and medicine (www.imperial.ac.uk/be-inspired/schools-outreach/secondary-schools/stem-programmes/pathways-to-medicine). Stellenbosch University has an extensive social impact programme (www.sun.ac.za/si/en-za/Pages/default.aspx), including an initiative to introduce high school students from disadvantaged communities to the Faculty of Medicine and Health Sciences (www.sun.ac.za/si/en-za/Pages/initiative.aspx?iid=1046)
Reference
https://doi.org/10.33424/FUTURUM270
BACTERIA – single-celled microorganisms, some of which affect humans in beneficial or harmful ways
TUBERCULOSIS (TB) – a potentially-fatal bacterial infection that mainly affects the lungs and usually causes coughing, fever and weight loss
MULTIDRUG-RESISTANT TUBERCULOSIS (MDR-TB) – a form of TB where the bacteria is resistant to the best anti-TB drugs
PAEDIATRICIAN – a medical doctor who diagnoses and treats health conditions in children
CLINICAL TRIAL – a type of scientific study used to test the effectiveness of a medical treatment
PLACEBO – a non-active treatment given to participants in clinical trials
In 2020, 1.1 million children worldwide were estimated to develop tuberculosis (TB), a potentially fatal disease that primarily affects the lungs and usually causes coughing, fever and weight loss. TB is caused by the bacteria Mycobacterium tuberculosis, and when someone with TB coughs, these bacteria are released into the air. If someone else breathes them in, these bacteria enter that person’s lungs, resulting in TB infection. Many people with TB infection never become unwell, as their immune system controls the bacteria in the body, but sometimes the number of bacteria increases, at which point the person develops TB disease and becomes unwell.
The good news is that doctors can test for TB infection before the onset of TB disease, enabling patients to be treated with drugs to ensure they do not progress to disease. However, some forms of TB bacteria have evolved to become resistant to the standard anti-TB drugs, resulting in multidrug-resistant (MDR) TB. If someone is infected with MDR-TB, normal treatments are ineffective, and doctors cannot prevent them developing the disease.
Dr James Seddon is a clinician scientist working as both a paediatrician and researcher, who divides his time between St Mary’s Hospital and Imperial College London in the UK, and Stellenbosch University in South Africa. James is conducting research into childhood TB in South Africa, to establish whether a new drug can prevent children who have been exposed to MDR-TB from developing TB disease. He also hopes to develop a gene expression test to identify children at higher risk of developing the disease, so treatments can be targeted more efficiently.
WHAT HAPPENS DURING A CLINICAL TRIAL?
James and his colleagues are running a clinical trial to test whether a new drug, levofloxacin, is safe and effective at preventing TB disease in children who have been exposed to MDR-TB. Normally, when someone is diagnosed with TB, the children in their household are given anti-TB drugs to prevent future TB. But if the person with TB is diagnosed with MDR-TB, then the normal drugs that are given to prevent future TB are ineffective and so no treatment can be given to children in the household.
James’s team contacts adults who have been diagnosed with MRD-TB. If they live with any children under the age of five, they are asked whether they would like the child to join the clinical trial. To determine whether levofloxacin prevents children exposed to MDR-TB infection developing TB disease, James must assess how many children progress to the disease when taking the drug, compared to how many progress to the disease without treatment.
Each child is randomly assigned to either receive levofloxacin tablets or a placebo (a dummy pill that doesn’t contain any treatment). “Neither the research team nor the families know which children are receiving levofloxacin treatment and which are receiving the placebo,” explains James. “This is important, because if people knew which medicine the child was receiving, it may influence how they are treated which in turn may affect the study results.” This is called a ‘double-blind’ clinical trial.
The children take the tablets for six months, during which time they are examined by the medical team every few weeks. The medical team continues to regularly assess every child for a year after they finish their treatment. Any children who develop TB disease will be diagnosed early and will receive the best possible treatment. “We hope that even the children receiving the placebo will be better off than children exposed to MDR-TB who are not in the trial,” says James.
WHAT RESULTS DOES JAMES EXPECT TO SEE?
“We expect fewer of the children who are given levofloxacin to progress to TB disease, compared to the children given the placebo,” says James. If this is the case, and assuming there are no adverse side effects of the drug, then this will be good evidence that levofloxacin is safe and effective at preventing TB disease in children exposed to MDR-TB. It will then be the responsibility of health policy makers to introduce this new drug to global health policies. “We would hope that if we find that levofloxacin reduces risk of TB disease progression and is safe, then the World Health Organization will recommend that, in future, all children who have been exposed to MDR-TB should be given levofloxacin,” James says.
HOW CAN A CHILD’S TB RISK BE DETERMINED?
Throughout the course of the trial, blood samples are taken from every child at regular intervals and tested to ensure the drug is safe. James and his colleagues are also analysing these blood samples to determine which genes are activated in each child at each point in time. By comparing the activated genes in children who progress to TB disease with the activated genes in children who remain well, James hopes to identify which activated genes can tell these two groups of children apart. A clinical test could then be developed so that when a child is exposed to TB, their risk of developing TB disease can be determined by testing for these few key activated genes.
This gene expression test would involve taking a small blood sample from the child by pricking their finger and analysing this blood in a machine that identifies the key activated genes within about half an hour. Children most at risk of developing TB disease could then be given appropriate treatment. This would enable countries with high rates of TB to prioritise their often-limited medical resources for children who most need them.
“If this kind of test were widely available, it could dramatically increase the number of children who are appropriately given treatment for TB infection and reduce the number of children who develop TB and who die from the disease each year,” says James.
 DR JAMES SEDDON
DR JAMES SEDDON
Consultant, Department of Paediatric Infectious Diseases, St Mary’s Hospital, London, UK
Reader in Global Child Health, Department of Infectious Diseases, Imperial College London, UK
Associate Professor, Desmond Tutu TB Centre, Stellenbosch University, South Africa
FIELD OF RESEARCH: Childhood Tuberculosis
RESEARCH PROJECT: Conducting a clinical trial to determine the effectiveness of levofloxacin at preventing TB disease in children and developing a gene expression test to identify children at risk of developing TB
FUNDER: Medical Research Council (MRC)
ABOUT CLINICIAN SCIENTISTS
A clinician scientist works as both a medical doctor treating patients and as a researcher in their specialist field. James is a paediatrician who looks after children with infectious diseases while also conducting research to find new tests and treatments for those diseases. He feels these roles complement each other well.
“The clinical work keeps me on my toes, making me aware of the most important questions that need answering to improve the care of children,” he says. “The research is very satisfying as it allows me to answer some of those questions, by changing the way we understand, diagnose and treat diseases.” But this dual role can also be challenging. “By doing two separate jobs, you feel that you are never giving either of them enough of your attention.”
WHAT DOES A CLINICIAN SCIENTIST’S DAY LOOK LIKE?
On clinical days, James visits his patients and works with health staff to decide what tests and treatments each child might need. He also advises other paediatricians about infectious diseases. He is often on-call overnight, ready to go into the hospital if he needs to attend to his patients.
On research days, James mainly does analysis and writing at his computer and sometimes sees children clinically who are taking part in his studies. Research projects can take years from start to finish, and they involve multiple stages: developing ideas and project proposals, applying for funding and ethics approval, collecting and analysing data, and finally, getting the research published. James always has lots of projects on the go, all at different stages. As research is a collaborative effort, this means he attends lots of meetings with his colleagues to discuss the progress of projects.
WHAT PERSONAL QUALITIES SHOULD CLINICIAN SCIENTISTS HAVE?
James says that anyone pursuing a career as a clinician scientist should be open minded and continually asking questions about the world, like ‘how can this be improved?’ or ‘how can we understand this better?’ Being organised and able to multitask are also useful skills. Clinical work and research are both team activities so it is vital that you can work well with other people.
EXPLORE A CAREER AS A CLINICIAN SCIENTIST
• It is important that clinician scientists are interested both in treating patients and in scientific research. The following articles written by clinician scientists highlight the rewards and challenges of the job:
– www.ncbi.nlm.nih.gov/pmc/articles/PMC4485885
– journals.biologists.com/dmm/article/3/3-4/125/2335/Interested-in-a-career-as-a-clinician-scientist
• University outreach programmes can help you learn more about both aspects of this career. Imperial College London offers activities for young people to learn about science (www.imperial.ac.uk/be-inspired/schools-outreach) and medicine (www.imperial.ac.uk/be-inspired/schools-outreach/secondary-schools/stem-programmes/pathways-to-medicine). Stellenbosch University has an extensive social impact programme (www.sun.ac.za/si/en-za/Pages/default.aspx), including an initiative to introduce high school students from disadvantaged communities to the Faculty of Medicine and Health Sciences (www.sun.ac.za/si/en-za/Pages/initiative.aspx?iid=1046)
PATHWAY FROM SCHOOL TO CLINICIAN SCIENTIST
• At school, science subjects such as biology and chemistry are necessary for studying medicine at university.
• James also studied English and history, which taught him to think critically, question ideas and communicate clearly, all of which are crucial skills for researchers. “I would suggest doing subjects that you are passionate about, that you are good at and that teach you to develop critical thinking,” he advises.
• As a medical doctor and a researcher, you must train and qualify in both these areas which will take several years. A degree in medicine takes five or six years in the UK (www.healthcareers.nhs.uk/explore-roles/doctors/training-doctor) and six years in South Africa (www.sun.ac.za/english/faculty/healthsciences/Pages/MBChB.aspx), both followed by many years of further clinical training to become a specialist. To become a research scientist (nationalcareers.service.gov.uk/job-profiles/research-scientist ), you will usually need to complete a PhD, which typically takes three to four years.
• James highlights that you don’t have to be a doctor to do research into health-related topics. Your clinical training could be in another field, such as nursing, pharmacy or psychology, which you could practice alongside your research. Or you could conduct medical research without being a clinical practitioner. In this case, degrees in biomedical science, biochemistry or molecular biology would be useful choices.
HOW DID JAMES BECOME A CLINICIAN SCIENTIST?
Medicine and research have been in my blood since I was young – my mother, grandfather and uncle were doctors, and my father and other grandfather were academics. My parents encouraged me think about other careers too, but medicine always felt like the right fit for me, and I continue to love it.
I did a great deal of sport when I was younger. I think participating in group activities (e.g., sport, music or drama) helps young people understand how to work in a team. Teamwork is crucial for both doctors and scientists. When applying for medical school, teamwork is considered an important life skill, so it is good to gain these experiences while at school.
I have a younger brother and sister, so grew up being familiar with how to understand and look after children. At medical school, I really enjoyed my paediatric placement and my decision to be a paediatrician was cemented while working in an emergency department in Australia. Working there, I enjoyed seeing children and their parents because it was usually lots of fun and always felt important.
I realised that childhood TB was an interesting area when I was working with Médecins sans Frontières (MSF) in Côte d’Ivoire. We looked after so many children with TB and HIV. I found it clinically very challenging as we had poor diagnostic tests and very limited drug options. I felt that this was a fascinating area that really needed more research.
Working for MSF was a real highlight of my career. It was hugely rewarding but also an enormously challenging experience. It was often scary to deliver medical care in unsafe situations with poor security and, having previously worked in wealthier countries, it was difficult to manage with so few resources. But it was also very satisfying to work with local staff to deliver healthcare where there were few other options. I learnt a great deal, both medically and from a leadership and logistics perspective.
Cape Town and London are two of the most amazing places in the world and I love being able to travel between the two. Most of my research takes place in Cape Town so I spend most of my time there. It is a beautiful place and it is wonderful to be able to do high quality research on a devastating disease like TB, while also being near mountains and beaches. I also love going back to London to work in the hospital, looking after children on the ward with complicated infections.
JAMES’S TOP TIPS
01 I think that the most important thing is to keep doing things that you find interesting and rewarding. Have confidence that they will take you somewhere worthwhile.
02 Always make sure you work with people you like, trust and respect. Life is too short to do otherwise!
Do you have a question for James?
Write it in the comments box below and James will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)








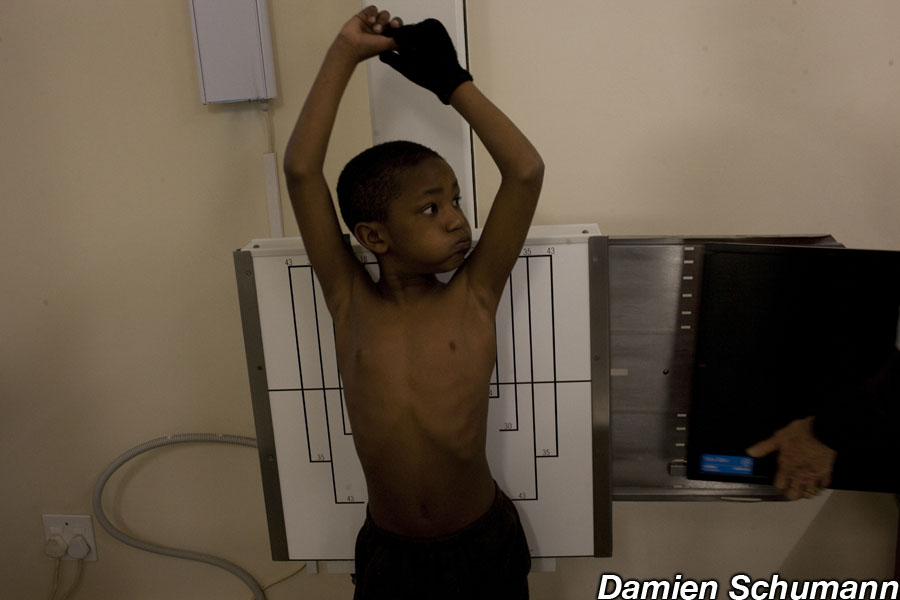











Congratulations Dr.James for finding Levofloxacin can prevent TB developing in children exposed to MDR TB .
God Bless you for finding a solution to a devastating scourge, which has made millions die prematurely. May it be taken up by WHO.