Could yeast and bacteria replace fossil fuels?
What if yeast and bacteria could replace fossil fuels? Dr Alan Goddard and his team are working on the MeMBrane Project, which aims to find ways of using microbes to make environmentally-friendly bioproducts such as biofuel
We are all aware of the potentially cataclysmic effect of continuing to rely on fossil fuels. While we might recycle our plastic, use electric cars or walk to school, efforts are being made to reduce our reliance in surprising quarters. One area where efficiency and a move away from fossil fuels is being considered is that of the use of microbes. Fossil fuel-based chemical production has been a historical tendency for some time and, while it has helped advance the field of biochemistry, it has had the unfortunate effect of releasing enormous amounts of carbon into the atmosphere. As we all now well know, these ‘non-renewable’ production methods are affecting the world’s climate. Now, there is a concerted effort to move away from such methods and instead look for alternatives that are cleaner, greener and more efficient, including biofuels.
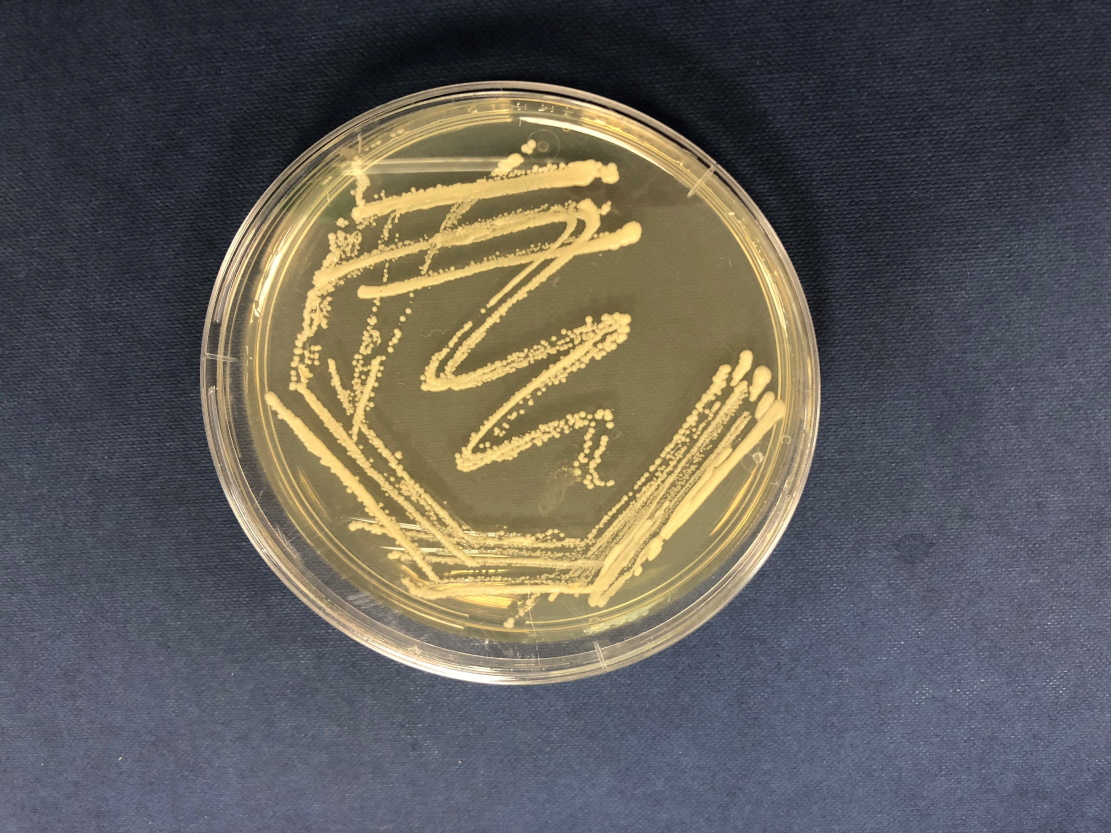
Based at Aston University in the UK, Dr Alan Goddard leads the MeMBrane project, which focuses on the production of what he and his team call cell factories. “Microbial cells can be grown on a variety of ‘foods’ including sugars, plant waste and even wood pulp,” explains Alan. “These sources of carbon are renewable and therefore more environmentally friendly. Bio-based routes to chemical production can also lead to purer products.”
TALK LIKE A BIOCHEMIST
CELL MEMBRANE – a biological membrane that separates the interior of all cells from the outside environment
MICROBE – a living thing that is too small to be seen with the naked eye
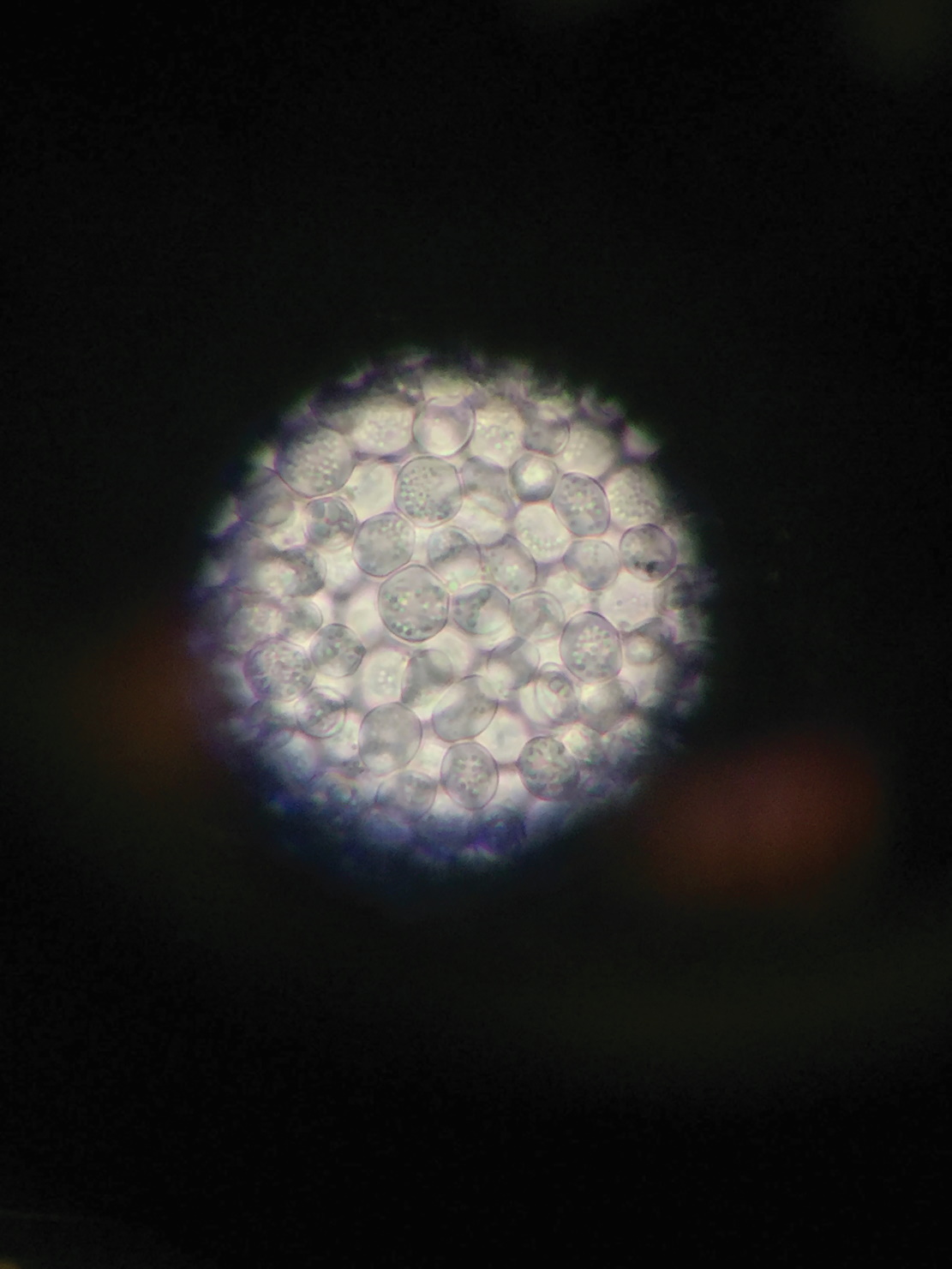
YEAST – a group of single-celled fungi that may reproduce by budding, i.e. when a bud detaches and forms into a new fungus
PROPIONIBACTERIUM – a gram-positive, anaerobic, rod-shaped genus of bacteria named for their unique metabolism
SYNTHETIC BIOLOGY – a field of science that involves redesigning organisms for useful purposes by engineering them to have new abilities
GREEN CHEMISTRY – an area of chemistry focused on designing products and processes that minimise or eliminate the use and generation of hazardous substances
BIOFUEL – a renewable energy source, made from organic matter or wastes, that can play a valuable role in reducing carbon dioxide emissions
WHAT KINDS OF MICROBIAL CELLS IS ALAN’S LAB WORKING WITH?
Alan and his team work with strains of Saccharomyces and Propionibacteria. Saccharomyces is a yeast that feeds on sugars and can be found on fruit, such as on the skin of grapes. Interestingly, this yeast has been domesticated to make the species Saccharomyces cerevisiae, commonly known as brewers’ or bakers’ yeast. Propionibacterium are present in a wide variety of environments. The Propionibacterium Alan and his team are most interested in are those found in dairy products such as cheese.
Ultimately, Alan and his team are interested in finding a means of overcoming our reliance on fossil fuels and are therefore investigating microbes as an alternative. “In our projects, Saccharomyces is used for the production of ethanol, whether that be for wine or for biofuels,” explains Alan. “Propionibacterium makes propionic acid, which can be used to keep yeast fermentations free from bacterial contamination.”
WHAT ARE SOME OF THE CHALLENGES INVOLVED IN ALAN’S RESEARCH?
The most obvious challenge is the production of ethanol by yeast. The hand gels we use in hospitals all contain ethanol, which helps to kill microbes – the ethanol breaks down the thin membrane around cells and kills them.
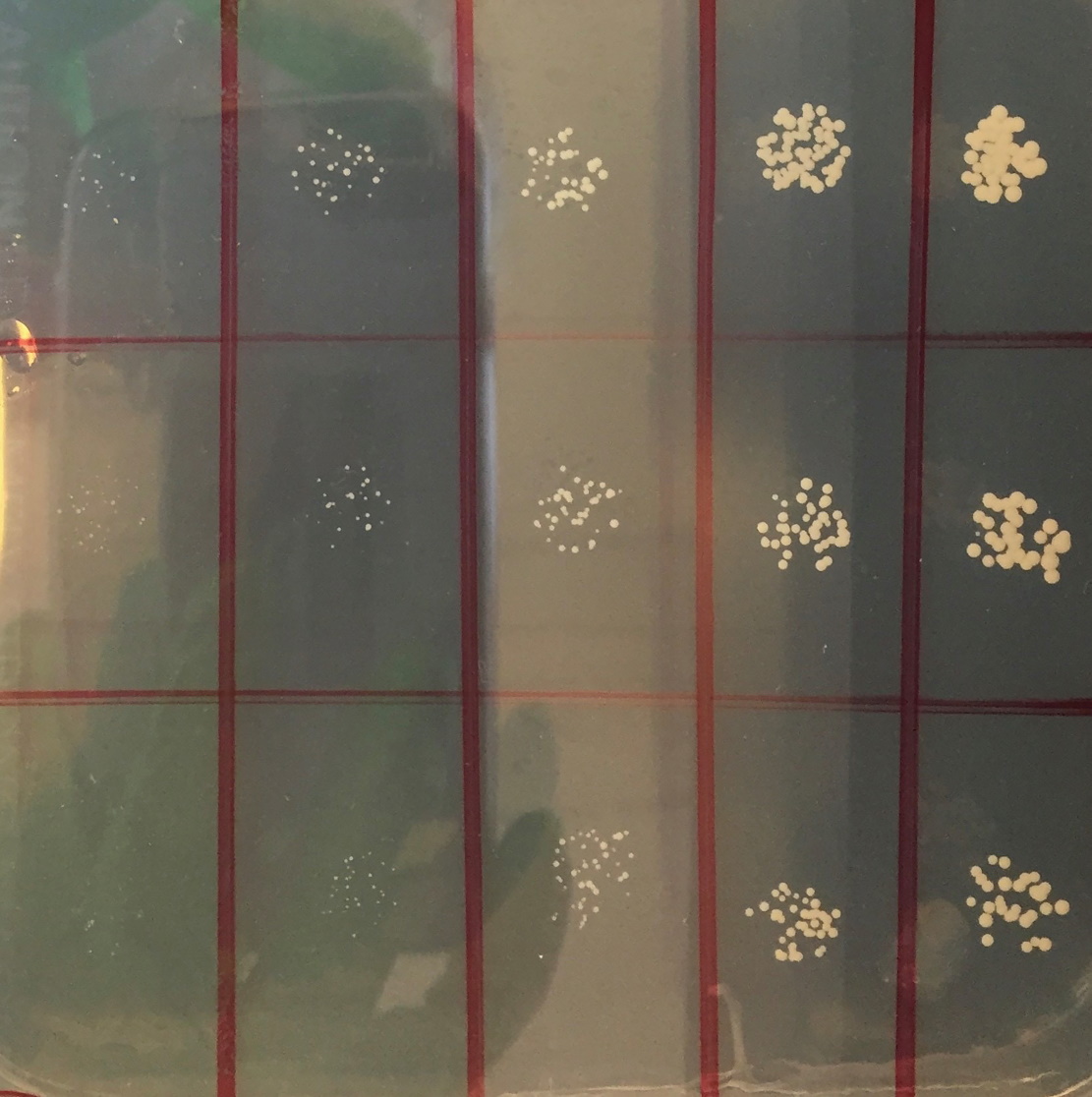
This is something that Alan and his team are battling with. “When the yeast produces the ethanol, it is killed at an alcohol content of 15%-16%, which explains why wine doesn’t ferment to a higher concentration,” explains Alan. “We would like to have yeast that is able to tolerate higher concentrations, both for the production of biofuels in a more cost-effective manner and also to add into fermentations, such as the secondary fermentation that champagne undergoes in the bottle.”
HAS ALAN’S LAB HAD ANY RECENT SUCCESSES?
Yes! The team has been working on a variety of different stresses that affect the cell membrane and they now understand the effects that ethanol and butanol, another type of alcohol, have on membranes at the molecular level. This allows the team to design strains that have the potential to be more resistant – these will be tested later this year, which could lead to some exciting results in the near future.
WHEN AND WHERE WILL THESE MICROBES REPLACE FOSSIL FUELS?
The short answer is soon and everywhere! As Alan points out, for decades, microbes were used to produce compounds such as butanol before fossil fuels took over. In many ways, the work of biochemists is to get back to where we once were, but the only way of doing that is to match the effectiveness and efficiency of fossil fuels. “One consideration is cost but reducing toxicity should help with this and as people become increasingly environmentally conscious this should help drive government policy,” explains Alan. “There is a lot of amazing work going on to get microbes to make chemicals they wouldn’t normally make, and this broadens their uses significantly.”
 DR ALAN GODDARD
DR ALAN GODDARD
Senior Lecturer in Biochemistry
Aston Membrane Proteins and Lipids (AMPL)
Aston University, UK
FIELD OF RESEARCH: Biochemistry
RESEARCH PROJECT: Alan’s work is focused on investigating Saccharomyces and Propionibacteria with a view to improving the tolerance of microbes. The team hopes to improve efficiency and product yield of engineered cell-based factories.
FUNDERS: UK Research and Innovation, European Research Area CoBioTech, H2020 Marie Skłodowska-Curie actions
Reference
https://doi.org/10.33424/FUTURUM50
Based at Aston University in the UK, Dr Alan Goddard leads the MeMBrane project, which focuses on the production of what he and his team call cell factories. “Microbial cells can be grown on a variety of ‘foods’ including sugars, plant waste and even wood pulp,” explains Alan. “These sources of carbon are renewable and therefore more environmentally friendly. Bio-based routes to chemical production can also lead to purer products.”
TALK LIKE A BIOCHEMIST
CELL MEMBRANE – a biological membrane that separates the interior of all cells from the outside environment
MICROBE – a living thing that is too small to be seen with the naked eye
YEAST – a group of single-celled fungi that may reproduce by budding, i.e. when a bud detaches and forms into a new fungus
PROPIONIBACTERIUM – a gram-positive, anaerobic, rod-shaped genus of bacteria named for their unique metabolism
SYNTHETIC BIOLOGY – a field of science that involves redesigning organisms for useful purposes by engineering them to have new abilities
GREEN CHEMISTRY – an area of chemistry focused on designing products and processes that minimise or eliminate the use and generation of hazardous substances
BIOFUEL – a renewable energy source, made from organic matter or wastes, that can play a valuable role in reducing carbon dioxide emissions
WHAT KINDS OF MICROBIAL CELLS IS ALAN’S LAB WORKING WITH?
Alan and his team work with strains of Saccharomyces and Propionibacteria. Saccharomyces is a yeast that feeds on sugars and can be found on fruit, such as on the skin of grapes. Interestingly, this yeast has been domesticated to make the species Saccharomyces cerevisiae, commonly known as brewers’ or bakers’ yeast. Propionibacterium are present in a wide variety of environments. The Propionibacterium Alan and his team are most interested in are those found in dairy products such as cheese.
Ultimately, Alan and his team are interested in finding a means of overcoming our reliance on fossil fuels and are therefore investigating microbes as an alternative. “In our projects, Saccharomyces is used for the production of ethanol, whether that be for wine or for biofuels,” explains Alan. “Propionibacterium makes propionic acid, which can be used to keep yeast fermentations free from bacterial contamination.”
WHAT ARE SOME OF THE CHALLENGES INVOLVED IN ALAN’S RESEARCH?
The most obvious challenge is the production of ethanol by yeast. The hand gels we use in hospitals all contain ethanol, which helps to kill microbes – the ethanol breaks down the thin membrane around cells and kills them.
This is something that Alan and his team are battling with. “When the yeast produces the ethanol, it is killed at an alcohol content of 15%-16%, which explains why wine doesn’t ferment to a higher concentration,” explains Alan. “We would like to have yeast that is able to tolerate higher concentrations, both for the production of biofuels in a more cost-effective manner and also to add into fermentations, such as the secondary fermentation that champagne undergoes in the bottle.”
HAS ALAN’S LAB HAD ANY RECENT SUCCESSES?
Yes! The team has been working on a variety of different stresses that affect the cell membrane and they now understand the effects that ethanol and butanol, another type of alcohol, have on membranes at the molecular level. This allows the team to design strains that have the potential to be more resistant – these will be tested later this year, which could lead to some exciting results in the near future.
WHEN AND WHERE WILL THESE MICROBES REPLACE FOSSIL FUELS?
The short answer is soon and everywhere! As Alan points out, for decades, microbes were used to produce compounds such as butanol before fossil fuels took over. In many ways, the work of biochemists is to get back to where we once were, but the only way of doing that is to match the effectiveness and efficiency of fossil fuels. “One consideration is cost but reducing toxicity should help with this and as people become increasingly environmentally conscious this should help drive government policy,” explains Alan. “There is a lot of amazing work going on to get microbes to make chemicals they wouldn’t normally make, and this broadens their uses significantly.”

DR ALAN GODDARD
Senior Lecturer in Biochemistry
Aston Membrane Proteins and Lipids (AMPL)
Aston University, UK
FIELD OF RESEARCH: Biochemistry
RESEARCH PROJECT: Alan’s work is focused on investigating Saccharomyces and Propionibacteria with a view to improving the tolerance of microbes. The team hopes to improve efficiency and product yield of engineered cell-based factories.
FUNDERS: UK Research and Innovation, European Research Area CoBioTech, H2020 Marie Skłodowska-Curie actions
ABOUT BIOCHEMISTRY
Biochemistry is about understanding the chemical processes that occur within all living things. While this obviously concerns humans, plants and animals, it is also focused on viruses and bacteria, or even the chemistry of digestion and bodily fluids.
The term biochemistry was coined in 1903 by a German scientist named Carl Neuberg. He is frequently referred to as the ‘father of modern biochemistry’ and pioneered research on fermentation. Much of his most important work is centred on examining and clarifying the metabolic complexities associated with a fermentation enzyme, known as zymase. Since then, there have been great leaps in the field of biochemistry. Scientists have recently developed the ability to make novel chemicals with microbes, which is likely to lead to new breakthroughs in the future and, like any breakthrough, what this will eventually give rise to is unknown – but isn’t that exciting?
WHAT COULD POSSIBLY BE THE NEXT BIG THING IN BIOCHEMISTRY?
Alan believes that new genetic tools such as CRISPR have helped to create excitement in the field. CRISPR (pronounced crisper) allows researchers to alter DNA sequences and therefore the function of genes. “I have been lucky that similar work to CRISPR has been routine in yeast for decades,” says Alan. “I think a future approach that has a huge amount of potential is coupling biology and chemistry. This enable microbes to make a ‘platform’ chemical that can then be modified by green chemistry approaches to make a variety of compounds.”
ARE THERE ANY FAMOUS BIOCHEMISTS?
Apart from the aforementioned ‘father of modern biochemistry’, Carl Neuberg, there is Isaac Asmiov, Louis Pasteur and the wonderfully named Novel Prizewinner Dorothy Crowfoot Hodgkin. All are worth reading more about, but one of the biochemists Alan admires is closer to home. “I have some colleagues that I really admire and they would be embarrassed if I named them; so, obviously I will!” says Alan. “Professor Roslyn Bill is an excellent scientist and colleague and does a huge amount to mentor early career researchers. It’s not just about the science, it’s about building a community of people who all work well together.”
And what about those Alan has not worked with? “Well, they aren’t particularly famous, but I am a big fan of Seymour Jonathan Singer and Garth L. Nicolson who developed what is known as the ‘fluid mosaic’ model of cell membranes,” says Alan. “They were the first to really propose how cell membranes function in the manner we understand them today and this underpins nearly all of the work in my lab.”
SHOULD YOU EMBARK ON A CAREER IN BIOCHEMISTRY?
Absolutely! Part of the process is deciding – or at least considering – whether you want to become a biochemist. Alan is of the firm belief that if you enjoy finding out how things work on a molecular level and then applying this to the real world, biochemistry is the field for you. “The interface of the physical and life sciences sets you up for a huge range of careers,” says Alan. “And there are plenty of opportunities in both academic and non-academic labs. For example, biotechnology is a rapidly-growing sector in the UK.”
HOW TO BECOME A BIOCHEMIST
• If you are genuinely interested in studying biochemistry, Alan highly recommends Aston University, where he is the Programme Director.
• The Biochemical Society is a dedicated resource to the field of biochemistry, which includes tonnes of useful info on how to develop a career in molecular bioscience.
• To keep abreast of developments in the field of biochemistry and biology, check out the Royal Society of Biology’s website. This year is the 10th anniversary of this important organisation and there is plenty to entertain, educate and inform.
• Biochemists can earn anywhere between £26,500 to £60,000 per annum in the UK, depending on their level of experience.
PATHWAY FROM SCHOOL TO BIOCHEMIST
You will usually need a science degree. Relevant degree subjects include: biochemistry, biotechnology, biopharmaceuticals, chemical and molecular biology, microbiology genetics and molecular biology.
https://nationalcareers.service.gov.uk/jobprofiles/biochemist
HOW DID DR ALAN GODDARD BECOME A BIOCHEMIST?
WHAT DID YOU IMAGINE YOURSELF DOING WHEN YOU WERE YOUNGER?
My parents were both teachers, so I think I was encouraged to stay in education from an early age. I was always interested in biology but probably didn’t know what I wanted to do beyond something to do with science and learning new things. I kind of fell into an academic career by following opportunities that arose.
WHO OR WHAT DREW YOU TO BIOCHEMISTRY?
I have always been interested in how organisms work on a molecular level. Essentially, all of life is biochemistry. I did my PhD working on yeast, although this focused more on molecular biology (working with DNA and making new strains). I then did research in the Biochemistry Department at the University of Oxford for about seven years, which opened my eyes to the wide variety of biochemistry out there.
HOW WOULD YOU DESCRIBE YOURSELF? ARE THESE CHARACTERISTICS USEFUL IN A CAREER AS A BIOCHEMIST?
I consider myself to be slightly obsessive, detail focused and I always ask questions! These characteristics definitely help, although you certainly don’t need all of them to be a biochemist. As long as you are interested in the science you will be fine!
WHAT DO YOU LOVE ABOUT YOUR WORK AND WHAT ARE YOU NOT SO KEEN ON?
Perhaps surprisingly, it is the same answer for both! No two days are the same, which is great in terms of being new and different and keeping you interested, but also bad in the sense that you never get to stand still and never really feel like you have everything under control!
WHAT ADVICE WOULD YOU GIVE TO YOUR YOUNGER SELF?
Be interested in a wide variety of things – you never know when one bit of information will be useful in a different context. One of my students once described me as having a “wide approximate knowledge” meaning that I know a little bit about a lot. Hopefully, there are some areas I also know a lot about!
ALAN’S TOP TIPS
1 If you want to become a biochemist, take biology and chemistry. Both of these subjects will prepare you to go on to bigger and better things!
2 It could also prove useful to study maths, not least because it is involved in so much of the sciences. However, a subject like history could also be a good idea, as biochemists do a surprising amount of writing!
3 Be persistent! No career is easy to get into but work hard and, most importantly, build your networks and be nice to people – you never know when they can help you out!