Does E really equal MC squared?
E=mc2 is arguably the most famous equation in the world, but is its description of the universe complete? Dr Matthew Redshaw, based at Central Michigan University, is the principal investigator of a project that is determined to find out
Albert Einstein developed his theory of special relativity in 1905 and still, some 115 years later, it forms an essential part of the basis of modern physics. While many of the mathematical and scientific calculations underpinning the theory are complex, the theory essentially explains how space and time are linked for objects moving at a consistent speed in a straight line. As an object approaches the speed of light – 186,282 miles per second – its mass becomes infinite and it cannot go any faster than the speed of light.
The theory of special relativity has many consequences, including the aforementioned universal speed limit and the mind-bending notion that time slows down for someone travelling close to the speed of light! But perhaps its most famous output is one of the most well-known equations in physics – E = mc2. It is fair to say that almost everyone has heard of this equation, but how many understand what it means? Put simply, it states ‘energy equals mass times the speed of light squared’. This is an extremely important statement, as it means that energy (E) and mass (m) are interchangeable and are therefore different forms of the same thing.
E = mc2 has been tested for its validity and accuracy countless times since Einstein published his theory, but now Dr Matthew Redshaw, based at Central Michigan University, USA is embarking on an ambitious project to perform the most precise direct test of the equation to date.
TALK LIKE A PHYSICIST
PROTONS – a particle with a positive charge
ELECTRONS – a particle with a negative charge
NEUTRONS – a particle with no charge
ISOTOPES – atoms that have the same number of protons and electrons, but a different number of neutrons
35CL – an isotope of chlorine with 17 protons and 18 neutrons
36CL – a naturally occurring radioactive isotope of chlorine
SPECIAL RELATIVITY – a physical theory regarding the relationship between space and time
GAMMA RAYS – a penetrating electromagnetic radiation arising from the radioactive decay of atomic nuclei
BLACK BODY – an object that absorbs all radiation (such as visible light, infrared light and ultraviolet light) that falls on it
WHAT TOOLS WILL BE USED TO TEST EINSTEIN’S THEORY?
With something that requires this level of precision, a lot of time and effort must go into building the right tools. The team is currently building a high precision Penning trap mass spectrometer (CHIP-TRAP) to conduct the research. “There are only a few other so-called Penning traps in the world capable of reaching the precision we are aiming for,” explains Matt. “What is unique about CHIP-TRAP is that it actually contains two traps. We use them like a balancing scale to compare the mass of two atoms against each other. This will help us perform these very precise mass measurements.”
HOW IS THE TEAM TESTING WHETHER E REALLY DOES EQUAL MC2?
To test the precision of Einstein’s theory, Matt and his team will be performing a very precise measurement of the mass difference between two types of chlorine atoms: Chlorine-35 (35Cl) and Chlorine-36 (36Cl). Because these isotopes have the same number of protons and electrons, they have the same chemical properties. However, 36Cl has one extra neuron – hence Matt’s decision to focus his research on these particular atoms. The difference in mass between the two atoms is not simply because of the extra neutron. Instead, as something explained by E = mc2, some of the additional mass of the extra neutron is given up as nuclear binding energy. So, the mass of 36Cl is actually less than the mass of 35Cl plus a neutron. By measuring the mass difference between 35Cl and 36Cl (and taking the mass of the additional neutron into account), Matt will be able to determine the neutron binding energy.
The final piece of the puzzle is that when a 35Cl nucleus captures a neutron to become 36Cl, it emits the excess binding energy in the form of gamma rays. A team of researchers in Grenoble, France, used the nuclear reactor there to capture neutrons on 35Cl. Then, they precisely measured the energy of the emitted gamma rays. By comparing the measurements for E with the mass difference, Matt and his team will be able to directly test E = mc2.
HOW FAR IS THE TEAM INTO THE RESEARCH?
Given the complexity of the CHIP-TRAP, there will not be any results this year. “We have been building the apparatus we will use to perform this measurement for five or six years now,” says Matt. “We are currently in the stage of trapping ions and developing our procedures and computer control system to perform the measurements. We hope to achieve the first results in the next couple of years.”
Ultimately, the results from the project will either increase confidence in the theory of relativity or, perhaps more excitingly, show that it is incomplete in describing our universe.
 DR MATTHEW REDSHAW
DR MATTHEW REDSHAW
Associate Professor
Department of Physics
College of Science and Engineering
Central Michigan University, USA
FIELD OF RESEARCH: Nuclear Physics
RESEARCH PROJECT: Matt’s work is concerned with attempting to perform a precise measurement of the mass difference between two types of chlorine atoms: Chlorine-35 and Chlorine-36. If successful, it would be the most precise direct test of Einstein’s famous E = mc2 equation to date.
FUNDERS: National Science Foundation; U.S. Department of Energy
This research is supported by the National Science Foundation and the US Department of Energy. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NSF or DoE.
ABOUT PHYSICS
E = mc2 is one of the key foundations in modern physics and is central to our understanding of the universe. However, many people do not understand what it means – so why is it so famous? “I think much of its fame is because it came from Einstein,” says Matt. “He was arguably the first physicist to reach celebrity in mainstream culture and his equation represents an idea from the mind of a genius.”
HOW DOES EINSTEIN’S EQUATION RELATE TO NUCLEAR PHYSICS?
Einstein’s theory shows that energy (E) and mass (m) are interchangeable – that mass is really just another form of energy. It tells us that we can measure the mass of an atom and learn something about its structure from how much energy is required to bind it together. It also tells us that there are huge quantities of energy stored inside the nucleus of an atom in the form of mass and that this energy can be released by splitting the atom, as is done in nuclear power plants and the atomic bomb.
ARE THERE ANY OTHER INTERESTING EQUATIONS RELATED TO ENERGY?
Matt is particularly fond of a deceptively simple looking equation that was developed by Max Planck towards the end of the 19th century. The equation is E = hf, where f is the frequency of light and h is Planck’s constant. “Planck introduced this equation as a mathematical trick to explain the discrepancy between the predicted and observed black body radiation spectrum,” explains Matt. “Einstein used this idea to explain another phenomenon – the photoelectric effect – that could not be explained with classic electromagnetic theory.”
Reference
https://doi.org/10.33424/FUTURUM52
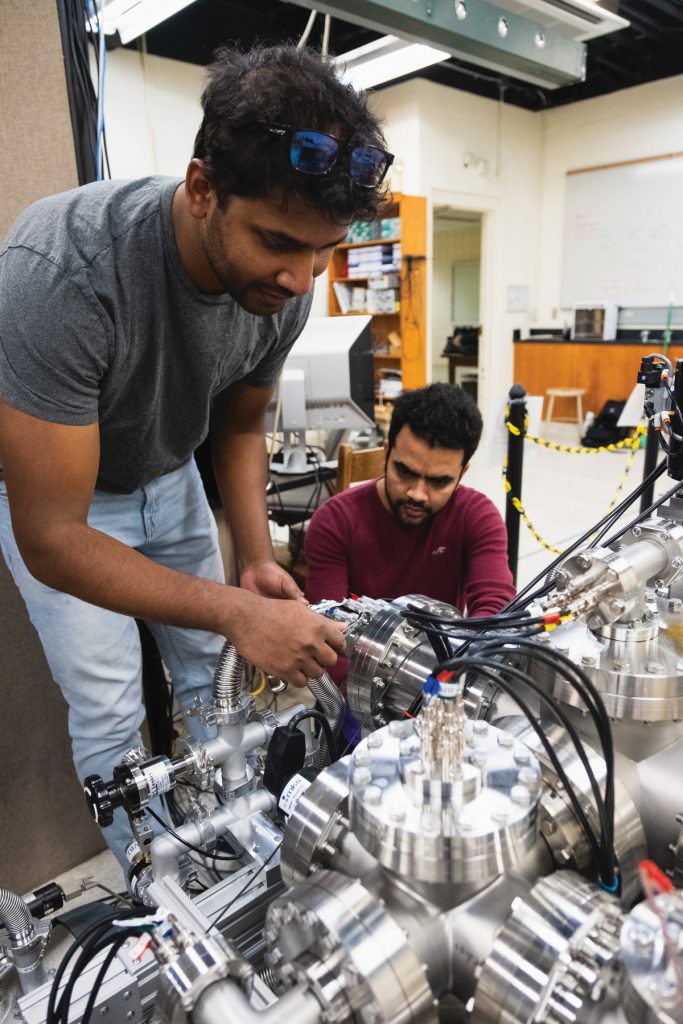
Philip Snoad, a visiting student from the University of Surrey on his year-long research placement, talks to Matt and two PhD students about his project.
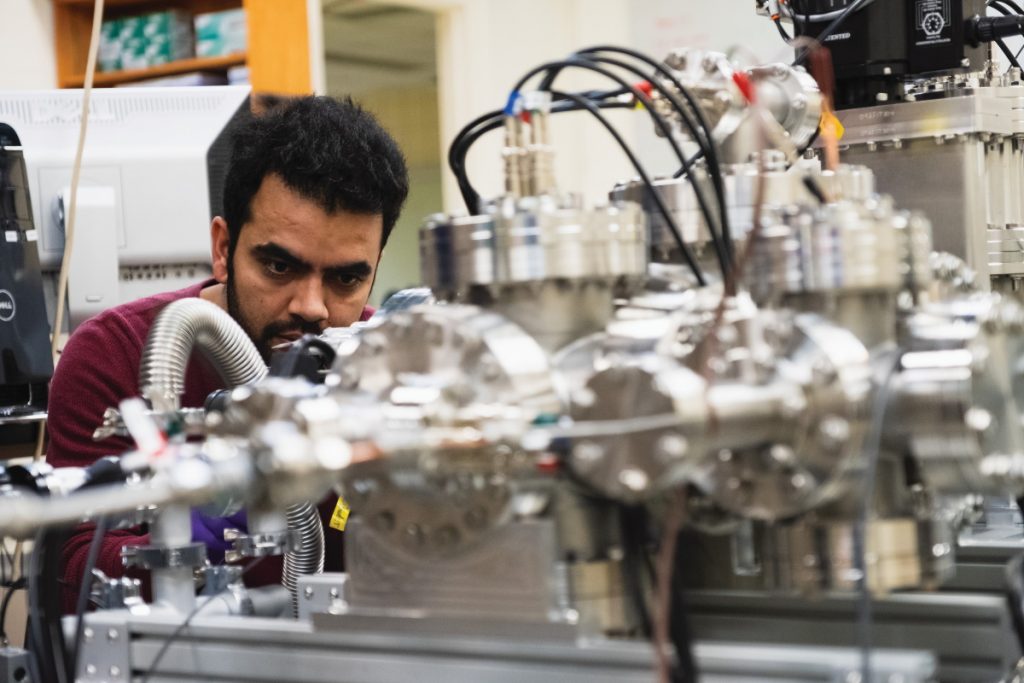
Although physicists need high level maths, physics and computer skills, experimental physics often requires skills you may have picked up working on your car or from playing with Meccano sets when you were a kid! Graduate students Nadeesha and Ramesh have spent many months in the lab designing and constructing the experimental apparatus shown here.
Matt in front of the 12 tesla superconducting magnet that forms the heart of the CHIP-TRAP apparatus that will be used to trap ions and measure their atomic mass.
The theory of special relativity has many consequences, including the aforementioned universal speed limit and the mind-bending notion that time slows down for someone travelling close to the speed of light! But perhaps its most famous output is one of the most well-known equations in physics – E = mc2. It is fair to say that almost everyone has heard of this equation, but how many understand what it means? Put simply, it states ‘energy equals mass times the speed of light squared’. This is an extremely important statement, as it means that energy (E) and mass (m) are interchangeable and are therefore different forms of the same thing.
E = mc2 has been tested for its validity and accuracy countless times since Einstein published his theory, but now Dr Matthew Redshaw, based at Central Michigan University, USA is embarking on an ambitious project to perform the most precise direct test of the equation to date.
TALK LIKE A PHYSICIST
PROTONS – a particle with a positive charge
ELECTRONS – a particle with a negative charge
NEUTRONS – a particle with no charge
ISOTOPES – atoms that have the same number of protons and electrons, but a different number of neutrons
35CL – an isotope of chlorine with 17 protons and 18 neutrons
36CL – a naturally occurring radioactive isotope of chlorine
SPECIAL RELATIVITY – a physical theory regarding the relationship between space and time
GAMMA RAYS – a penetrating electromagnetic radiation arising from the radioactive decay of atomic nuclei
BLACK BODY – an object that absorbs all radiation (such as visible light, infrared light and ultraviolet light) that falls on it
WHAT TOOLS WILL BE USED TO TEST EINSTEIN’S THEORY?
With something that requires this level of precision, a lot of time and effort must go into building the right tools. The team is currently building a high precision Penning trap mass spectrometer (CHIP-TRAP) to conduct the research. “There are only a few other so-called Penning traps in the world capable of reaching the precision we are aiming for,” explains Matt. “What is unique about CHIP-TRAP is that it actually contains two traps. We use them like a balancing scale to compare the mass of two atoms against each other. This will help us perform these very precise mass measurements.”
HOW IS THE TEAM TESTING WHETHER E REALLY DOES EQUAL MC2?
To test the precision of Einstein’s theory, Matt and his team will be performing a very precise measurement of the mass difference between two types of chlorine atoms: Chlorine-35 (35Cl) and Chlorine-36 (36Cl). Because these isotopes have the same number of protons and electrons, they have the same chemical properties. However, because 36Cl has one – hence Matt’s decision to focus his research on these particular atoms. The difference in mass between the two atoms is not simply because of the extra neutron. Instead, as something explained by E = mc2, some of the additional mass of the extra neutron is given up as nuclear binding energy. So, the mass of 36Cl is actually less than the mass of 35Cl plus a neutron. By measuring the mass difference between 35Cl and 36Cl (and taking the mass of the additional neutron into account), Matt will be able to determine the neutron binding energy.
The final piece of the puzzle is that when a 35Cl nucleus captures a neutron to become 36Cl, it emits the excess binding energy in the form of gamma rays. A team of researchers in Grenoble, France, used the nuclear reactor there to capture neutrons on 35Cl. Then, they precisely measured the energy of the emitted gamma rays. By comparing the measurements for E with the mass difference, Matt and his team will be able to directly test E = mc2.
HOW FAR IS THE TEAM INTO THE RESEARCH?
Given the complexity of the CHIP-TRAP, there will not be any results this year. “We have been building the apparatus we will use to perform this measurement for five or six years now,” says Matt. “We are currently in the stage of trapping ions and developing our procedures and computer control system to perform the measurements. We hope to achieve the first results in the next couple of years.”
Ultimately, the results from the project will either increase confidence in the theory of relativity or, perhaps more excitingly, show that it is incomplete in describing our universe.
 DR MATTHEW REDSHAW
DR MATTHEW REDSHAW
Associate Professor
Department of Physics
College of Science and Engineering
Central Michigan University, USA
FIELD OF RESEARCH: Nuclear Physics
RESEARCH PROJECT: Matt’s work is concerned with attempting to perform a precise measurement of the mass difference between two types of chlorine atoms: Chlorine-35 and Chlorine-36. If successful, it would be the most precise direct test of Einstein’s famous E = mc2 equation to date.
FUNDERS: National Science Foundation; U.S. Department of Energy
This research is supported by the National Science Foundation and the US Department of Energy. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NSF or DoE.
ABOUT PHYSICS
E = mc2 is one of the key foundations in modern physics and is central to our understanding of the universe. However, many people do not understand what it means – so why is it so famous? “I think much of its fame is because it came from Einstein,” says Matt. “He was arguably the first physicist to reach celebrity in mainstream culture and his equation represents an idea from the mind of a genius.”
HOW DOES EINSTEIN’S EQUATION RELATE TO NUCLEAR PHYSICS?
Einstein’s theory shows that energy (E) and mass (m) are interchangeable – that mass is really just another form of energy. It tells us that we can measure the mass of an atom and learn something about its structure from how much energy is required to bind it together. It also tells us that there are huge quantities of energy stored inside the nucleus of an atom in the form of mass and that this energy can be released by splitting the atom, as is done in nuclear power plants and the atomic bomb.
ARE THERE ANY OTHER INTERESTING EQUATIONS RELATED TO ENERGY?
Matt is particularly fond of a deceptively simple looking equation that was developed by Max Planck towards the end of the 19th century. The equation is E = hf, where f is the frequency of light and h is Planck’s constant. “Planck introduced this equation as a mathematical trick to explain the discrepancy between the predicted and observed black body radiation spectrum,” explains Matt. “Einstein used this idea to explain another phenomenon – the photoelectric effect – that could not be explained with classic electromagnetic theory.”
Planck’s equation formed part of a revolution of physics, where scientists began to understand that the rules of the incredibly small were different to those of the massive. This led to our beginning to understand quantum physics and the development of quantum mechanics, which continues to lead to tremendous advances in a range of fields.
WHAT ABOUT OTHER NOTABLE PHYSICISTS?
Richard Feynman is one of Matt’s favourite physicists. Feynman was an American theoretical physicist who did important work relating to quantum mechanics and quantum electrodynamics. “I loved reading his books during my teenage years and beyond. Like Einstein, he acquired somewhat of a celebrity status and is an icon to many physicists,” says Matt. “He had such unique insights into physics problems and won the Nobel prize for his role in developing quantum electrodynamics. He was also a fantastic storyteller and interesting character.”
SHOULD YOU EMBARK ON A CAREER IN NUCLEAR PHYSICS?
Only you can answer that, but the subject is so varied that it can lead to a wide range of different career paths. Nuclear physics is often seen as something otherworldly almost, but the field helps to answer some fundamental questions. “A career in nuclear physics could involve doing research to answer questions such as ‘How is the matter we see around us – and that we are made of – produced in stars?’,” says Matt. “Then there is the opportunity to work on cleaner and safer ways to produce nuclear energy or developing medical treatments to fight cancer.”
• The Facility for Rare Isotope Beams (FRIB) is currently being built at Michigan State University and is scheduled to begin operating in 2022. Matt says that dozens of universities around the US are involved in developing equipment to use at FRIB and are planning experiments to run. This will create a lot of opportunities for students and physicists to get involved.
• If you are interested in becoming a physicist, UCAS has a dedicated page that breaks down all the possible pathways, opportunities and where to find out more.
• According to Glassdoor, the average salary for a physicist in the US is $95,391.
PATHWAY FROM SCHOOL TO PHYSICIST
Aspiring physicists usually major in physics from the time they are undergraduate students, building a thorough foundation of physics knowledge by studying science and mathematics courses such as electromagnetism, thermodynamics, quantum mechanics and optics.
https://www.degreequery.com/what-degree-do-i-need-to-become-a-physicist/
HOW DID DR MATTHEW REDSHAW BECOME A PHYSICIST?
WHAT DID YOU WANT TO BE WHEN YOU WERE YOUNGER?
When I started secondary school, I wanted to be an engineer because I liked maths and science and that seemed like an obvious path. But that was before I really knew what a career as a scientist could look like!
WHO OR WHAT DREW YOU TO NUCLEAR PHYSICS?
It was really astronomy and then astrophysics that drew me to nuclear physics. I was always fascinated with the night sky; a particularly notable memory is of being woken up in the night by my parents in 1986 to see Halley’s comet. I also loved the awesome pictures of stars, galaxies and other objects that were being taken by the Hubble telescope. In my physics classes, I learned that it is nuclear physics that fuels the life and death of stars. I think it is really cool that by studying the laws of physics that describe how the tiny nucleus of an atom behaves, you can understand the universe on a galactic scale.
WHAT DO YOU LOVE MOST ABOUT THE WORK YOU DO?
What I love most about it, is that I get to do a lot of different things. I get to teach and mentor students, from first year undergraduates just starting out on their physics journey, to PhD students who are developing the skills to start on their careers as independent scientists. I also get to think about interesting problems and ideas in physics, and to plan and run experiments to help solve those problems and test those ideas. This can involve building equipment in the lab, collecting data during an experiment, writing computer code to analyse the data, and talking and writing about those results.
IF YOU COULD GO BACK IN TIME, WHAT ADVICE WOULD YOU GIVE TO YOUR YOUNGER SELF?
I would tell myself not to think that you can’t be good at something just because you don’t know how to do it yet. You really can learn new skills with practice and hard work. Don’t put it off. Get to work! And get help – there are many teachers and mentors who want to help. Especially if they see you are putting in the effort to succeed.
MATT’S TOP TIPS
1 The advice I give my students is to get involved in some research. Pick a university that emphasises student involvement in research. This could be through an undergraduate research project as part of your degree, a research placement at a lab or in industry, or working for a professor during the summer.
2 Take as many science and maths classes as you can. If you have the opportunity, take any computing and programming classes that are available. Having the knowledge and skills to use computers to run experiments and to analyse data is vital.
3 Concentrate on mastering the fundamentals of maths and physics like calculus and mechanics. All the advanced courses you take will build on these concepts.









Please consider the Atomic Hydrogen Torch and the work of Nikola Tesla. Look it up.
Nothing can stop this from getting out, so you might as well report about it.
E DOES NOT = MC^2.
We have it all.
Articles, and esp science or technical subjects articles without the date of creation or last modification are kind of useless
Thank you for your feedback George. Indeed you are correct and will notice all new content is now uploaded with a published date.