Fuel cells: A new way to power the world
Currently, the way we power cars, trucks and other vehicles is reliant on the burning of fossil fuels, so attention has shifted to other energy sources such as batteries and electrochemical fuel cells. Dr Mark Tuckerman, Professor of Chemistry and Mathematics at New York University, leads a team that is particularly interested in anion exchange membrane fuel cells.
WHAT ARE ELECTROCHEMICAL FUEL CELLS?
A fuel cell is an electrochemical device capable of delivering power by converting the chemical energy stored in a fuel into electricity. Common fuel sources that are used include methanol and hydrogen. When methanol is used, the fuel cells produce carbon dioxide, which is undesirable; in contrast, when hydrogen fuel is used, the only waste products are water – which can be recycled back into the fuel cell – and heat. Hydrogen is difficult to produce efficiently, but a number of research groups are making new inroads into this problem.
Fuel cells can be used to power anything that runs on electricity, so they are ideal for common devices such as computers and mobile phones. They can also be used to power vehicles, where efficiencies reach 40-60% compared to the approximately 20% efficiency of typical petrol-powered engines. The heat generated by fuel cells can also be used to heat up homes, large apartment complexes, or office buildings and factories.
WHAT IS THE ADVANTAGE OF THE ANION EXCHANGE MEMBRANE FUEL CELL OVER OTHER ELECTROCHEMICAL FUEL CELLS?
In order to convert the chemical energy of the fuel into electricity, a fuel cell uses a pair of chemical reactions known as reduction oxidation or “redox” reactions. Under normal conditions, these reactions can be quite slow, which makes the energy conversion process inefficient. In order to accelerate the chemical reactions, a catalyst is needed. “Platinum has proved to be an effective catalyst for redox reactions,” says Mark. “Its use in the acidic conditions in which many fuel cells operate is preferred because it is particularly unreactive and less susceptible to corrosion under these conditions.”
Alkaline fuel cells operate under basic conditions using a different set of redox reactions, which are their key advantage. Although a catalyst is still required, these operating conditions allow for catalysts to be constructed from cheaper metals, making the alkaline fuel cell a better candidate for mass production.
HOW DO ANION EXCHANGE FUEL CELLS WORK?
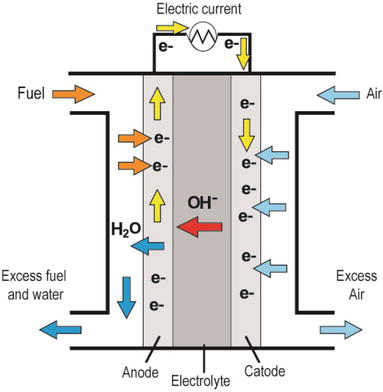
The basic operation of an anion exchange fuel cell is relatively simple. As with any electrochemical device, an anion exchange fuel cell consists of two metal electrodes, a positively charged anode and a negatively charged cathode, and an ion-conducting material between them known as an electrolyte. The two electrodes are also joined by an electrical circuit through which electrons produced in a chemical reaction at the anode flow to produce an electric current, driven by a voltage applied across the circuit.
Source: S. Castañeda Ramírez and R. Ribandeneira Paz in New Trends in Ion Exchange Studies, InTech Open Series (DOI:10.5772/ intechopen.77148), 2018.
Reference
https://doi.org/10.33424/FUTURUM22
Currently, the way we power cars, trucks and other vehicles is reliant on the burning of fossil fuels, so attention has shifted to other energy sources such as batteries and electrochemical fuel cells. Dr Mark Tuckerman, Professor of Chemistry and Mathematics at New York University, leads a team that is particularly interested in anion exchange membrane fuel cells.
WHAT ARE ELECTROCHEMICAL FUEL CELLS?
A fuel cell is an electrochemical device capable of delivering power by converting the chemical energy stored in a fuel into electricity. Common fuel sources that are used include methanol and hydrogen. When methanol is used, the fuel cells produce carbon dioxide, which is undesirable; in contrast, when hydrogen fuel is used, the only waste products are water – which can be recycled back into the fuel cell – and heat. Hydrogen is difficult to produce efficiently, but a number of research groups are making new inroads into this problem.
Fuel cells can be used to power anything that runs on electricity, so they are ideal for common devices such as computers and mobile phones. They can also be used to power vehicles, where efficiencies reach 40-60% compared to the approximately 20% efficiency of typical petrol-powered engines. The heat generated by fuel cells can also be used to heat up homes, large apartment complexes, or office buildings and factories.
WHAT IS THE ADVANTAGE OF THE ANION EXCHANGE MEMBRANE FUEL CELL OVER OTHER ELECTROCHEMICAL FUEL CELLS?
In order to convert the chemical energy of the fuel into electricity, a fuel cell uses a pair of chemical reactions known as reduction oxidation or “redox” reactions. Under normal conditions, these reactions can be quite slow, which makes the energy conversion process inefficient. In order to accelerate the chemical reactions, a catalyst is needed. “Platinum has proved to be an effective catalyst for redox reactions,” says Mark. “Its use in the acidic conditions in which many fuel cells operate is preferred because it is particularly unreactive and less susceptible to corrosion under these conditions.”
Alkaline fuel cells operate under basic conditions using a different set of redox reactions, which are their key advantage. Although a catalyst is still required, these operating conditions allow for catalysts to be constructed from cheaper metals, making the alkaline fuel cell a better candidate for mass production.
HOW DO ANION EXCHANGE FUEL CELLS WORK?
The basic operation of an anion exchange fuel cell is relatively simple. As with any electrochemical device, an anion exchange fuel cell consists of two metal electrodes, a positively charged anode and a negatively charged cathode, and an ion-conducting material between them known as an electrolyte. The two electrodes are also joined by an electrical circuit through which electrons produced in a chemical reaction at the anode flow to produce an electric current, driven by a voltage applied across the circuit.

Source: S. Castañeda Ramírez and R. Ribandeneira Paz in New Trends in Ion Exchange Studies, InTech Open Series (DOI:10.5772/ intechopen.77148), 2018.
HOW IS COMPUTER MODELLING HELPFUL FOR IMPROVING AND MODIFYING THESE FUEL CELLS?
The key to the successful operation of an alkaline fuel cell is the creation of AEMs that can transport the hydroxide ions as efficiently as possible. Experimentally making and testing all the possible AEMs is both wasteful and extremely time consuming. By using a computational model, however, Mark and his team can explore a large space of AEM design parameters simply by tweaking a few numbers and repeating calculations – a considerably easier task. Therefore, computational investigation of the performance of different possible AEMs allows the parameter space to be narrowed down to a few key values that can be passed on to synthetic chemists. This means that synthetic chemists have a much smaller set of polymers to make and test, and the resulting hydrogen fuel cells could go to market much more quickly.
Mark explains: “To give you an idea of what can be investigated computationally, we can predict the morphology [form or structure] of a given polymer architecture, estimate the rate of hydroxide transport, and study the chemical stability of the material in the presence of the alkaline solution in which it is immersed.” These predictions can be tested against experimental results, which then help to refine the computational models. The improved models, in turn, become better guides for the synthesis, and so on. This type of feedback loop can be a powerful route to the rapid design of new functional materials such as AEMs and to the discovery of general optimal design principles for these materials.
 Dr Mark Tuckerman
Dr Mark TuckermanProfessor of Chemistry and Mathematics, New York University, USA
FIELD OF RESEARCH: Theoretical Chemistry
RESEARCH PROJECT: Mark’s research is focused on exploring the potential of anion exchange membrane fuel cells as a renewable energy technology. One of the chief aims of his work is to arrive at a set of fundamental design principles that can be used to generate a variety of anion exchange membranes exhibiting high hydroxide conductivity. Achieving this goal could ultimately result in high-performing hydrogen fuel cells with the potential to revolutionise how the world powers a wide variety of vehicles and instruments.
FUNDER: National Science Foundation (Division of Materials Research)
 Dr Mark Tuckerman
Dr Mark TuckermanProfessor of Chemistry and Mathematics New York University, USA
FIELD OF RESEARCH: Theoretical Chemistry
RESEARCH PROJECT: Mark’s research is focused on exploring the potential of anion exchange membrane fuel cells as a renewable energy technology. One of the chief aims of his work is to arrive at a set of fundamental design principles that can be used to generate a variety of anion exchange membranes exhibiting high hydroxide conductivity. Achieving this goal could ultimately result in high-performing hydrogen fuel cells with the potential to revolutionise how the world powers a wide variety of vehicles and instruments.
FUNDER: National Science Foundation (Division of Materials Research)
From an eagle-eye view, theoretical science exists to give a higher sense of order to experimental observations as they are presently known, or to point to new observations that can be experimentally verified. Without theory, experimental science is merely a catalogue of measured results. More specifically, to understand what theoretical chemistry is, it is important to position it within the wider context of chemistry as a whole. Because chemistry is, in essence, the science of molecules, their interactions and transformations – and molecules are nothing more than assemblies of atoms whose behaviour is governed by the fundamental laws of physics – it is possible to approach chemical problems from a theoretical physical perspective.
Thus, theoretical chemistry applies the basic equations of physics to atomic assemblies to reveal how atoms arrange to form molecular structures and how they move in chemical reactions to cause molecular transformations. Such atomic level information can provide insights into chemical processes that cannot be accessed experimentally. As these equations are enormously complicated, obtaining analytical, mathematical solutions is generally not possible, which is why high-performance computers and sophisticated algorithms must be used.
WHAT BENEFITS DOES THEORETICAL CHEMISTRY BRING TO SOCIETY?
The synergy that exists between theory and experiment means that the resources and amount of time needed to solve a particular problem can be reduced. Theoretical chemistry is now an integral and indispensable part of chemical research and plays a major role in bringing the benefits of scientific research to society as a whole, including areas such as medicine, clean energy, electronics and advanced materials.
WHY SHOULD YOUNG PEOPLE STUDY THEORETICAL CHEMISTRY?
Theoretical chemistry trains students in skills that are applicable in both academia and industry, and that are transferable beyond the molecular sciences to an increasingly technically-orientated job market. In order to work in theoretical chemistry, students need to be familiar with advanced mathematical methods and statistics, be skilled in programming and software design and, depending on their particular research area, have a good understanding of biology, physics, computer science or engineering, and, of course, chemistry.
IF A STUDENT IS INTERESTED IN A CAREER IN THEORETICAL CHEMISTRY, WHERE WOULD BE THE BEST PLACE TO START?
A career in theoretical chemistry requires an advanced university degree in the subject, meaning, at the very least, a Master’s degree or, far better, a PhD. Therefore, gaining admission to a graduate programme is essential. Generally, the best undergraduate degrees from which to springboard into a graduate programme in theoretical chemistry are physics, chemistry, chemical engineering, applied mathematics or biology.
WHAT OPPORTUNITIES ARE AVAILABLE FOR THEORETICAL CHEMISTS?
Academic research is not the only career path for someone with an advanced degree in theoretical chemistry. Computational chemists are needed at pharmaceutical and chemical companies and are also highly sought after in scientific software developments companies. As mentioned above, the skills learned by theoretical chemists are highly transferable to non-scientific fields, including finance, data science, research administration and science policy.
• The American Chemical Society says that the median salary for theoretical chemists is $100,000.
• There are a wide range of paid positions for students seeking summer internship opportunities. You can work closely with a senior scientist to boost your skills and knowledge.
• Internetchemistry.com provides lots of resources on computational and theoretical chemistry, including software, lecture notes and tutorials.
WHO INSPIRED YOU TO STUDY THE SCIENCES WHEN YOU WERE AT SCHOOL?
While I learned science from many truly excellent professors during my undergraduate years, I was largely inspired by an abstract admiration for the giants of physics – Einstein, Feynman, Bohr, Schrödinger, Heisenberg, etc. – and a desire to understand, both conceptually and mathematically, what they had achieved.
YOUR UNDERGRADUATE DEGREE AND PHD ARE IN PHYSICS, YET YOU ARE A PROFESSOR OF CHEMISTRY AND MATHEMATICS, AND MUCH OF YOUR RESEARCH INVOLVES COMPUTER MODELLING. WHY DID YOU CHANGE FIELDS?
As an undergraduate physics major, I was doing research in theoretical particle physics. While I thoroughly enjoyed this type of abstract, highly mathematical research (and still do), I maintained a strong tangential interest in molecular science, perhaps because it was easier to see its broad and immediate applications. When I got to graduate school, I waivered a bit between these two areas, but when I came to realise just how many of the things I had learned in my study of theoretical physics were also applicable in theoretical and computational chemistry, the choice became much clearer. It was actually possible for me to work with the abstract methods I had learned as a theoretical physicist and apply them in an area that seemed imminently practical!
DID THIS CHANGE IN FIELDS REQUIRE A LOT OF RE-TRAINING AND LEARNING?
I suppose there was a learning curve to ascend in changing fields, shoring up the gaps in my chemistry knowledge, and retraining my mind to look at things in the way chemists do. I was surprised at how much of a language barrier there was, at that time, between the physics and chemistry communities, which required me to learn a new vocabulary. However, I pushed myself to quickly become conversant in this vocabulary because I knew that, as a newly minted assistant professor of chemistry, I would need it to do something I had never done before – teach undergraduate chemistry courses! In terms of my research, which has a significant overlap with physics, there was not such a chasm to bridge; indeed, the gap between physics and chemistry has narrowed further over the years since I made the transition. However, I am ultimately glad that
I started out in physics because the training it provided ultimately proved invaluable for my work in theoretical chemistry. Moreover, I suspect it is easier to make the switch from physics to chemistry than it would be to do the reverse.
FINALLY, WHAT IS THE MOST ENJOYABLE PART OF YOUR JOB?
It’s very difficult to say – it’s like asking: “What is your favourite part of a chocolate sundae or a tiramisu?” Imagine a career in which you have complete freedom to work on whatever interests you and experience the thrill of discovery as part of your job description, and you’ll have a good idea of what being an academic researcher is like. This might well be the best job there is, even if it isn’t that remunerative financially. Every day is different and stimulating in some way. There are opportunities to travel and have stimulating discussions with colleagues all over the world. Best of all is the opportunity this job affords to shape the next generation of scientists. Seeing one’s students and postdocs mature as researchers, watching them come up with their own ideas, and witnessing their thrill of discovery when their ideas finally work – of all that this job entails, this aspect may be the most rewarding!
1 Earning an advanced degree in theoretical chemistry requires hard work and commitment. If you wish to gain a PhD, it will probably take between five and six years beyond your undergraduate degree. While it can be frustrating and humbling, ultimately it will prove very rewarding.
2 If you are majoring in chemistry, be sure to enrol in mathematics and physics courses beyond those that are required. Courses to choose include multivariable calculus, linear algebra, differential equations, classical and quantum mechanics, and statistical mechanics.
3 It will also be highly beneficial to learn one (or more) programming languages. Particularly useful choices are Python and C++. Do try and get involved in undergraduate research, too – the practical training and perspective on research you will gain from the experience will be invaluable.






