How can diabetes lead to vision loss, and how can this be prevented?
Diabetic retinopathy is the leading cause of blindness among working-age adults in the developed world. Dr David Antonetti, a molecular and cell biologist at the University of Michigan, USA, works in the field of ophthalmology to investigate how diabetes causes eye issues. He hopes his research will lead to the development of new treatments for diabetes-related vision loss.
TALK LIKE AN OPHTHALMOLOGIST
Blood-retinal barrier — a physiological barrier that regulates the flow of nutrients, ions and molecules between the bloodstream and retina
Cultured cell — a cell grown in a laboratory
Cytokine — a small protein molecule released from one cell that signals a cell response in another cell, usually by binding to a specific receptor
Neuron — a nerve cell that conveys a signal by electrical response
Ophthalmology — the branch of medicine focused on the eye and vision
Permeability — the ability of a material to allow substances to pass through it. A material with high permeability allows substances to pass through easily
Phosphorylation — the addition of a phosphate group (PO43-) to a molecule
Physiological — relating to the normal functioning of an organism
Retina — the thin layer of light-detecting cells at the back of the eye
Signalling — the process of a cell emitting, receiving and processing instructions
Diabetes is a chronic health condition in which the body cannot control its blood sugar levels properly. You have probably heard of diabetes, but you might not associate it with vision loss. However, diabetes can affect the retina – the thin layer of light-detecting cells at the back of the eye – which can lead to diabetic retinopathy.
What is diabetic retinopathy?
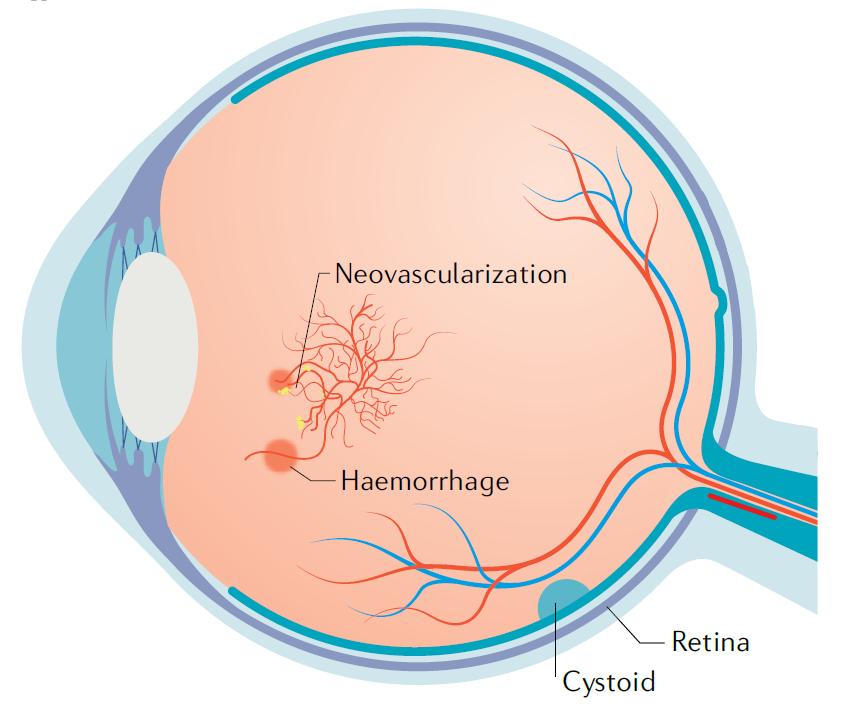
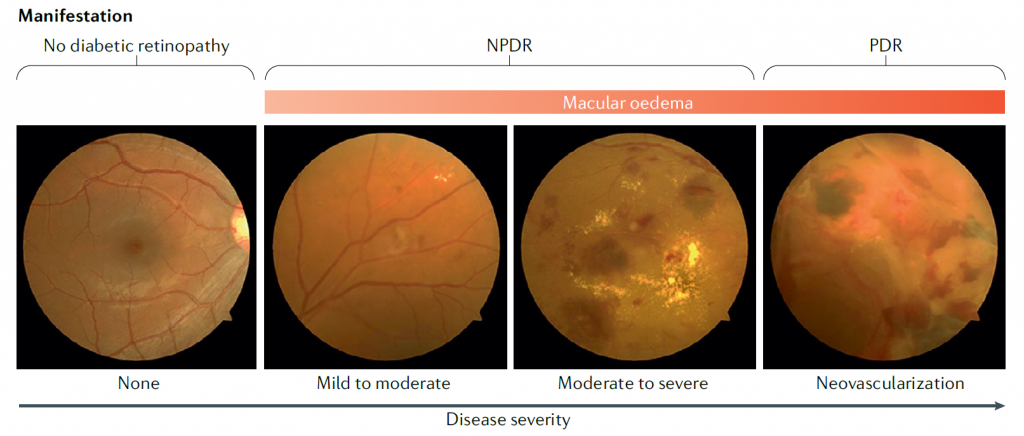
Diabetic retinopathy is characterised by changes in the retinal blood vessels, and symptoms include blurry, spotted or total loss of vision. A third of all people with diabetes have diabetic retinopathy, and severe forms of the disease are the leading cause of blindness in working-age adults. Dr David Antonetti, a molecular and cell biologist at the University of Michigan, hopes to improve our understanding of the mechanisms behind the condition and develop new therapies to treat the disease.
The exact cause of diabetic retinopathy is still unknown, but it appears that factors associated with diabetes, such as increased blood glucose (sugar) and fat, alter the normal retinal environment. “Changes in the retina include a loss of neurons, inflammation, leaky blood vessels and abnormal blood vessel growth,” explains David. If retinal blood vessels leak, fluid can accumulate in the retina which reduces a person’s vision. If abnormal blood vessels grow, they can pull the retina away from its support within the eye, leading to complete vision loss.
What role does the blood-retinal barrier play in diabetic retinopathy?
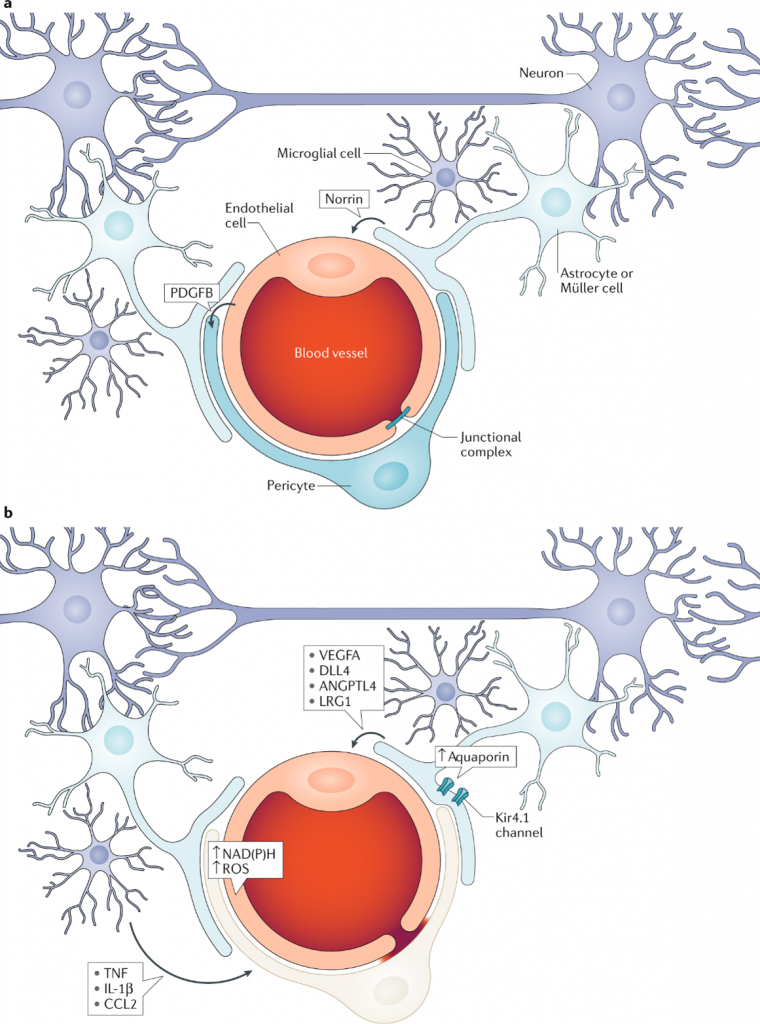
We experience vision because neurons in the retina direct light that enters our eyes. The retina includes a series of neurons that relay light signals to the brain by allowing the flow of ions across the neuron cell membrane. For the brain to convert these signals into vision, the neurons in the brain and retina require a highly specialised environment.
To maintain this neural environment, a blood-retinal barrier exists in our eyes and a blood-brain barrier exists in our brain. These physiological barriers regulate the flow of small molecules into and out of the retina and brain without disturbing neural signalling. The barriers are formed from blood vessel cells and controlled by tight junction proteins, which either prevent or allow certain substances to pass through. “Tight junction proteins help seal the blood vessel cells together and play a role in controlling which molecules can pass from the blood into the neural environment,” explains David.
Diabetes can increase the permeability of the blood-retinal barrier. This means more molecules can pass from the blood into the retina, including substances that should not enter the neural environment, leading to vision loss.
What is David investigating?
During diabetes, the permeability of the blood-retinal barrier is altered by cytokines, small protein molecules that signal cell responses. These cytokines impact the tight junction proteins responsible for maintaining the blood-retinal barrier. David’s lab is studying two cytokines that play significant roles in diabetic retinopathy – vascular endothelial growth factor (VEGF) and norrin.
VEGF stimulates the growth of blood vessel cells and causes retinal blood vessels to become leaky, while norrin promotes the organisation of tight junction proteins creating the blood-retinal barrier. “Our lab is studying how VEGF signals blood vessel leakiness and how norrin signals blood vessel tightness,” says David.
How does David study cytokine signalling?
To understand cytokine signalling in blood vessel cells, David’s team collects animal eyes from the local butcher. “We remove the retinas, isolate the small blood vessels from them, then grow blood vessel cells (called endothelial cells) from these blood vessels,” says David. David and his team then add VEGF to these cultured cells to induce leakiness or norrin to promote barriers, allowing them to explore changes in tight junctions in the presence of different cytokines.
By using very high-resolution microscopes, the team can observe whether the tight junctions change location or create gaps that allow substances to pass through the blood-retinal barrier. “We grow the cells on a filter and use fluorescently labelled molecules to trace the rate of flux across the cells,” explains David. “This allows us to measure the tightness or leakiness of the cells after different treatments.”
Once David has identified the factors that regulate cellular processes, he studies them in more detail using genetically engineered mouse models. “Mice are a better model for the human eye than cultured cells as we can test disease response,” he explains. By altering a mouse’s genetic code, David creates mice that express or lack specific cytokines or proteins. He then makes the mice diabetic and observes whether their retinal blood vessels leak and how their vision is affected. “We cannot ask the mice to read an eye chart!” David says. “However, we can observe how they respond to a visual cue. For example, if we move a pattern of black and white vertical lines in front of a mouse, it will reflexively follow the pattern. We can assess its vision by making the lines smaller until the mouse no longer sees the pattern.”
What has David discovered?
Reference
https://doi.org/10.33424/FUTURUM395
“A major accomplishment in our lab has been identifying phosphorylation of occludin as a key process in diabetic retinopathy,” says David. “Occludin is a protein that regulates the tight junction, either making a tight barrier between cells or allowing the blood vessels to leak.” Phosphorylation is the addition of a phosphate group (PO43-) to a molecule and is a common mechanism of cell signalling. Phosphorylation of occludin acts like a switch, signalling tight junction proteins to move away from the membrane of blood vessel cells and into the cells themselves. This increases the permeability of the blood-retinal barrier, causing leaks.
David and his team discovered this by genetically altering their cultured cells so they contained a mutant version of occludin that could not be phosphorylated. These cells no longer became leaky when responding to VEGF. Next, David introduced the mutant occludin into diabetic mice. “We found that the non-phosphorylated mutant occluding greatly reduced the ability of VEGF to make blood vessels leak,” says David. “Importantly, adding this mutant form of occludin to mice preserved the blood-retinal barrier when they had diabetes and completely prevented loss of vision.”
This is a significant breakthrough. David’s research reveals that maintaining the blood-retinal barrier preserves visual function in diabetes, helping scientists to understand how current therapies blocking VEGF work and highlighting barrier promoting factors, like norrin, as a potential new therapy.
How are David’s discoveries relevant to stroke research?
A stroke can occur if a blood clot blocks a blood vessel in the brain, causing a part of the brain to die. David’s research into the blood-retinal barrier during diabetic retinopathy has led to unexpected discoveries about the blood-brain barrier during a stroke.
“Having learnt the importance of occludin to the blood-retinal barrier, we collaborated with colleagues to ask whether this same protein and its phosphorylation affected the blood-brain barrier during a stroke,” says David. By inducing strokes in mice, David and his colleagues observed that the mice showed signs of occludin phosphorylation in the brain blood vessels surrounding the region of the stroke. They then induced strokes in genetically engineered mice that had mutant occludin. In this case, the team observed that leakage of the brain blood vessels was greatly reduced during a stroke.
“The only current therapy for a stroke is providing an enzyme that breaks up the blood clot. However, if given too late, this enzyme makes the stroke worse by causing severe bleeding in the brain,” says David. “Working with stroke experts, we found that blocking occludin phosphorylation can prevent this bleeding.” This means drugs that prevent this phosphorylation may now be explored as a means to prevent brain bleeding when treating stroke patients, highlighting the range of important clinical outcomes from David’s research.
 Dr David Antonetti
Dr David Antonetti
Department of Ophthalmology and Visual Sciences, Kellogg Eye Center, University of Michigan, USA
Fields of research: Ophthalmology, Molecular and Cell Biology
Research project: Investigating factors that affect the blood-retinal barrier during diabetic retinopathy
Funders: US National Institutes of Health (NIH), Research to Prevent Blindness (RPB)
About ophthalmology
Ophthalmology is the study of eyes and vision. Clinical ophthalmologists are medical doctors who specialise in the diagnosis and treatment of eye disorders. They are experts in all aspects of eye care, from how to prevent eye diseases to different treatment options for different eye conditions. In contrast, vision research scientists, like David, conduct scientific research to understand how the eye functions. “Vision research scientists investigate how our eyes allow vision and how vision can be affected by disease,” explains David. “These are the scientists who are trying to find new cures to prevent or treat blindness and vision loss.”
The excitement of research
“The most rewarding part of my job is working with others and making truly novel discoveries,” says David. “There is a great deal of excitement in learning something new about how our cells work.” David and his team have made many significant discoveries over the years, from determining that tight junctions are dynamic entities regulated by phosphorylation ‘switches’ that open and close the blood-retinal barrier, to uncovering the fact that norrin can reverse the effects of diabetes and restore the blood-retinal barrier. “Sometimes, when you are very lucky, these discoveries have a profound effect on the clinical community and can impact patient care or provide novel treatment options.”
The importance of collaboration for scientific discoveries
“Discoveries involve interactions with other people,” says David. “One person never drives the whole process.” For example, while David is a molecular and cell biologist, the success of his lab comes from the team members’ range of disciplinary backgrounds, such as vision science and biomedical science, and their collaboration with researchers from other laboratories. “By listening to novel perspectives and valuing all voices, we can reach new ideas that none of us could have achieved individually.”
Pathway from school to ophthalmology
• At school, study biology, chemistry, physics and mathematics.
• Your university pathway will depend on your ophthalmology direction. To become a clinical ophthalmologist, you will need to complete a medical degree and qualify as a medical doctor, then complete specialised training in the field of ophthalmology. To become a vision research scientist, complete a degree in a subject such as biology, molecular biology, biochemistry, biomedical science or vision science, then specialise in a laboratory focused on vision research in your postgraduate training.
• David recommends taking courses in cell biology to learn how cells signal and interact with each other, and courses in mathematics and computing to learn how to process data. “Modern approaches to science use large and complex datasets,” he says. “It is important to understand how to manage these data.”
• David recommends reading to discover which aspects of vision you are most passionate about. “Read articles in magazines and newspapers that talk about new research findings,” he advises. “Follow scientists on Twitter and read about their work.” While academic publications can be very technical, many scientists also write articles for a public audience that will be easier to follow. For example, learn more about diabetic eye diseases with this Futurum article: www.futurumcareers.com/reaching-out-to-overcome-diabetic-eye-disease-in-the-philippines
• If possible, try to find work experience in a lab dedicated to research in vision.
• As a clinical ophthalmologist, you will work with patients to diagnose and treat their eye conditions. The American Academy of Ophthalmology has an informative page about the different types of eye care provider (e.g., ophthalmologist, optometrist, optician) and the qualifications needed by each profession: www.aao.org/eye-health/tips-prevention/what-is-ophthalmologist
• As a vision research scientist, you will work in a scientific lab to conduct experiments to improve scientific understanding of vision. You could be an academic researcher in a university lab, like David, or an industrial researcher in a company lab. “There are lots of ways to succeed in the field of research, as there are many different ways of conducting research,” says David.
Meet David
Who inspired you to become a scientist?
When I was younger, I was always curious and interested in all aspects of science. My parents were both teachers and encouraged discovery. My mother, particularly, encouraged me to ask questions. In my second year of college, I had a great cell biology teacher, who described the excitement and joy he felt when making a new discovery in research. That lecture confirmed my wish to become a research scientist.
What successes have you had in your career?
I have been lucky to work with outstanding people in my laboratory and have had success obtaining funding and publishing manuscripts that describe our research. Publishing research manuscripts is critical for scientists, as the research is reviewed by other experts in the field, then made available to the broader scientific community if it makes important conclusions. Other researchers build on these published findings, so it is an important step in the research process. The publishing and funding success of my lab has put me in a position to help others in our department work towards a successful scientific career.
You have won numerous awards for research and teaching. What do these recognitions mean to you?
I received the ‘Jules Francois prize for young investigator in ophthalmology’ from the Ophthalmologia Belgica and the ‘Most inspirational teacher’ award for graduate education at Penn State University. Research can be hard, with many setbacks and great ideas that are proven wrong. In fact, I might say the ability to accept when data clearly reveals that an idea you loved turns out to be wrong, is one of the most important qualities to have as a scientist! Getting recognition along the way is very helpful for seeing the bigger picture.
What is your favourite fact about the eye?
‘It’s impossible to sneeze with your eyes open.’ I don’t really know if it is true. But, as a scientist, I love that people will test the hypothesis and try to prove it wrong.
What do you enjoy doing in your free time?
Spending time with my wife, going on walks in nature, playing rock guitar and watching University of Michigan sports.
David’s top tips
1. Think about your future and what you want to achieve. It takes a plan and commitment to become successful.
2. Follow your passion.
Do you have a question for David?
Write it in the comments box below and David will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)