How can vaccinating pigs protect people?
AMPLIFICATION – when a virus infecting a particular species can replicate itself extremely rapidly
ANTIBODY – a protein used by the immune system to prevent viruses infecting cells
HOST – an organism within which a virus resides
IMMUNE SYSTEM – the complex network of biological processes which organisms use to protect themselves from diseases
PATHOGEN – an organism that causes disease
T CELLS – white blood cells that destroy infected cells
VACCINE – a substance containing molecules or weakened pathogens, which stimulates the body’s immune system to prevent or control real infections more effectively in the future
VIRUS – a microscopic structure which replicates itself by invading living cells, usually causing damage to the host
ZOONOTIC DISEASE – an infectious disease that jumped from animals to humans
In 1998, Nipah virus (NiV) was first identified following infection of pig farmers in Malaysia. While the disease can be mild in pigs, if the virus transfers to a human host, it is far more dangerous. Initial symptoms in humans include fever, headache, coughing and breathing difficulties, followed by brain swelling leading to a coma. “The lack of specific symptoms in pigs makes NiV hard to diagnose, so it can spread quickly,” says Dr Rebecca (Becca) McLean, a vaccinologist at The Pirbright Institute. “If a human becomes infected with NiV, the chance of death varies from 45% to 75%.” While the original outbreak of NiV was contained by 1999, the danger it presents has not disappeared.
HOW CAN NIPAH VIRUS BE CONTAINED?
NiV passed to pigs from wild fruit bats, which are native to Southeast Asia, Africa and Australia. NiV has been detected in wild bat populations in Malaysia, Bangladesh, India, Singapore and Thailand. “When pigs live in close proximity to fruit bats, there is a chance that NiV can be transmitted to them through the bat’s urine, faeces or saliva,” says Professor Simon Graham. “When pigs become infected, they can amplify the virus. This can cause humans who have contact with these animals to become infected.”
To contain the 1998 NiV outbreak, over a million pigs were slaughtered in Malaysia and, to this day, pig farming is still banned in many parts of the country. The outbreak had devastating consequences for Malaysia’s economy as the government had to pay over US$500 million in compensation to farmers who lost their jobs and livestock.
To eliminate the threat of a future NiV outbreak requires an effective vaccination against the virus. It would not be possible to vaccinate wild populations of fruit bats, so Becca, Simon and their colleagues are developing a vaccine to prevent the spread of NiV in pigs.
HOW WOULD A NIPAH VIRUS VACCINE WORK?
To protect itself against infection, the body’s immune system must learn to identify and destroy viruses. When NiV infects a cell, it uses two proteins, named glycoprotein (G) and fusion protein (F). Vaccinologists can include these proteins in vaccines to ‘teach’ the immune system to fight NiV before it enters the body.
By replicating the exact G or F proteins unique to NiV, a successful vaccine would activate two important elements of the immune system – Y-shaped proteins named antibodies and white blood cells named T cells. “T cells have a role in not only destroying virus-infected cells, but also in helping other cells of the immune system to respond better,” says Becca. “Antibodies can bind to the NiV G and F proteins, preventing the virus from infecting cells.” The vaccine would therefore teach cells how to identify and destroy the real virus, without becoming infected by it.
HOW DO BECCA AND SIMON TEST VACCINES?
Becca, Simon and their colleagues are developing and testing three different NiV vaccine candidates to determine which is most effective at preventing NiV. One vaccine contains the NiV G protein, one contains the NiV F protein and the third delivers the NiV G protein using a weakened form of a virus, known as a ‘vaccine vector’. The specific vaccine vector they are testing is ‘ChAdOx1’, which was also recently used as the basis for the AstraZeneca COVID-19 vaccine.
To test the vaccines, the team immunised pigs with two doses of each vaccine candidate and then collected blood samples to analyse the immune responses they initiated. Becca and Simon discovered that each vaccine offers its own unique advantages. While antibody responses to the NiV G protein were best at preventing the virus from infecting cells, the NiV F protein was most effective in preventing the virus from spreading from cell-to-cell, and the ChAdOx1 vector stimulated the strongest T cell responses (Figure 1).
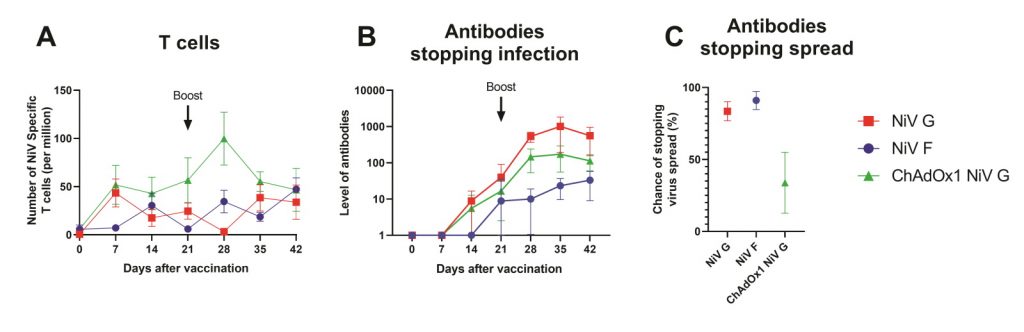
Figure 1: NiV vaccine candidates induce different immune responses in pigs. A) ChAdOx1 NiV G (green) produced the greatest T cell response. B) NiV G (red) produced the antibody response best able to prevent infection of cells. C) NiV F (blue) produced the best antibody response to limit the spread of NiV between cells (fusion).
Three weeks later, the team exposed pigs to real NiV. This allowed Becca and Simon to compare the protection provided by each vaccine. “Despite the differences in immune responses between the three vaccine candidates, all three vaccines provided a comparable high level of protection,” explains Simon (Figure 2).
Figure 2: All three vaccines protect pigs against NiV. Alongside pigs which remained unvaccinated, we experimentally infected pigs vaccinated with our three vaccines. We then took swabs from the nose to see if any virus could be found there. Very minimal amounts of virus could be detected from the vaccinated pigs, but lots of virus could be found in the pigs that were not vaccinated. This suggests that the vaccinated pigs cannot transmit NiV.
These results are incredibly promising, and researchers can now consider how vaccines could be used to prevent and control future NiV outbreaks. This may include emergency ‘ring vaccination’ campaigns, where pigs surrounding an outbreak are immunised, or routine vaccinations of pig populations to prevent outbreaks from arising in the first place.
WHAT CHALLENGES STILL NEED TO BE OVERCOME?
Although Becca, Simon and their colleagues have shown their vaccines are effective, vaccination campaigns cannot yet be carried out. To determine the success of a vaccination campaign, researchers would need to carefully monitor virus neutralising antibodies in the blood of pigs. Currently, however, there is no way to tell the difference between pigs which gained these antibodies from a vaccine, and those that were infected with the real virus.
“Unless a new diagnostic test is developed which can distinguish between infected and vaccinated animals, a NiV vaccine for pigs could not be used for routine vaccination, as it would interfere with surveillance for the virus,” explains Becca. Therefore, the team is now focused on designing this test.
If successful, the team can begin conversations with local governments and vaccine regulators about how the vaccine could be rolled out in pig populations. Ultimately, these efforts are an important part of the wider work of vaccinologists. Many other viruses in wild animal populations have the potential for zoonosis. We will not know which ones can successfully infect humans until it is too late, so the work of future generations of vaccinologists will be crucial to prepare for this eventuality.
 DR REBECCA MCLEAN
DR REBECCA MCLEANPROFESSOR SIMON GRAHAM
The Pirbright Institute, UK
FIELD OF RESEARCH: Vaccinology
RESEARCH PROJECT: Developing a vaccine against Nipah virus in pigs
PROJECT PARTNERS: University of Oxford, UK; Australian Centre for Disease Preparedness, Australia; University of Queensland, Australia; International Centre for Diarrhoeal Disease Research, Bangladesh; Zoetis, USA
FUNDERS: This research is funded by the Department of Health and Social Care using UK Aid funding and is managed by Innovate UK. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health and Social Care.
Reference
https://doi.org/10.33424/FUTURUM255
AMPLIFICATION – when a virus infecting a particular species can replicate itself extremely rapidly
ANTIBODY – a protein used by the immune system to prevent viruses infecting cells
HOST – an organism within which a virus resides
IMMUNE SYSTEM – the complex network of biological processes which organisms use to protect themselves from diseases
PATHOGEN – an organism that causes disease
T CELLS – white blood cells that destroy infected cells
VACCINE – a substance containing molecules or weakened pathogens, which stimulates the body’s immune system to prevent or control real infections more effectively in the future
VIRUS – a microscopic structure which replicates itself by invading living cells, usually causing damage to the host
ZOONOTIC DISEASE – an infectious disease that jumped from animals to humans
In 1998, Nipah virus (NiV) was first identified following infection of pig farmers in Malaysia. While the disease can be mild in pigs, if the virus transfers to a human host, it is far more dangerous. Initial symptoms in humans include fever, headache, coughing and breathing difficulties, followed by brain swelling leading to a coma. “The lack of specific symptoms in pigs makes NiV hard to diagnose, so it can spread quickly,” says Dr Rebecca (Becca) McLean, a vaccinologist at The Pirbright Institute. “If a human becomes infected with NiV, the chance of death varies from 45% to 75%.” While the original outbreak of NiV was contained by 1999, the danger it presents has not disappeared.
HOW CAN NIPAH VIRUS BE CONTAINED?
NiV passed to pigs from wild fruit bats, which are native to Southeast Asia, Africa and Australia. NiV has been detected in wild bat populations in Malaysia, Bangladesh, India, Singapore and Thailand. “When pigs live in close proximity to fruit bats, there is a chance that NiV can be transmitted to them through the bat’s urine, faeces or saliva,” says Professor Simon Graham. “When pigs become infected, they can amplify the virus. This can cause humans who have contact with these animals to become infected.”
To contain the 1998 NiV outbreak, over a million pigs were slaughtered in Malaysia and, to this day, pig farming is still banned in many parts of the country. The outbreak had devastating consequences for Malaysia’s economy as the government had to pay over US$500 million in compensation to farmers who lost their jobs and livestock.
To eliminate the threat of a future NiV outbreak requires an effective vaccination against the virus. It would not be possible to vaccinate wild populations of fruit bats, so Becca, Simon and their colleagues are developing a vaccine to prevent the spread of NiV in pigs.
HOW WOULD A NIPAH VIRUS VACCINE WORK?
To protect itself against infection, the body’s immune system must learn to identify and destroy viruses. When NiV infects a cell, it uses two proteins, named glycoprotein (G) and fusion protein (F). Vaccinologists can include these proteins in vaccines to ‘teach’ the immune system to fight NiV before it enters the body.
By replicating the exact G or F proteins unique to NiV, a successful vaccine would activate two important elements of the immune system – Y-shaped proteins named antibodies and white blood cells named T cells. “T cells have a role in not only destroying virus-infected cells, but also in helping other cells of the immune system to respond better,” says Becca. “Antibodies can bind to the NiV G and F proteins, preventing the virus from infecting cells.” The vaccine would therefore teach cells how to identify and destroy the real virus, without becoming infected by it.
HOW DO BECCA AND SIMON TEST VACCINES?
Becca, Simon and their colleagues are developing and testing three different NiV vaccine candidates to determine which is most effective at preventing NiV. One vaccine contains the NiV G protein, one contains the NiV F protein and the third delivers the NiV G protein using a weakened form of a virus, known as a ‘vaccine vector’. The specific vaccine vector they are testing is ‘ChAdOx1’, which was also recently used as the basis for the AstraZeneca COVID-19 vaccine.
To test the vaccines, the team immunised pigs with two doses of each vaccine candidate and then collected blood samples to analyse the immune responses they initiated. Becca and Simon discovered that each vaccine offers its own unique advantages. While antibody responses to the NiV G protein were best at preventing the virus from infecting cells, the NiV F protein was most effective in preventing the virus from spreading from cell-to-cell, and the ChAdOx1 vector stimulated the strongest T cell responses (Figure 1).

Figure 1: NiV vaccine candidates induce different immune responses in pigs. A) ChAdOx1 NiV G (green) produced the greatest T cell response. B) NiV G (red) produced the antibody response best able to prevent infection of cells. C) NiV F (blue) produced the best antibody response to limit the spread of NiV between cells (fusion).
Three weeks later, the team exposed pigs to real NiV. This allowed Becca and Simon to compare the protection provided by each vaccine. “Despite the differences in immune responses between the three vaccine candidates, all three vaccines provided a comparable high level of protection,” explains Simon (Figure 2).

Figure 2: All three vaccines protect pigs against NiV. Alongside pigs which remained unvaccinated, we experimentally infected pigs vaccinated with our three vaccines. We then took swabs from the nose to see if any virus could be found there. Very minimal amounts of virus could be detected from the vaccinated pigs, but lots of virus could be found in the pigs that were not vaccinated. This suggests that the vaccinated pigs cannot transmit NiV.
These results are incredibly promising, and researchers can now consider how vaccines could be used to prevent and control future NiV outbreaks. This may include emergency ‘ring vaccination’ campaigns, where pigs surrounding an outbreak are immunised, or routine vaccinations of pig populations to prevent outbreaks from arising in the first place.
WHAT CHALLENGES STILL NEED TO BE OVERCOME?
Although Becca, Simon and their colleagues have shown their vaccines are effective, vaccination campaigns cannot yet be carried out. To determine the success of a vaccination campaign, researchers would need to carefully monitor virus neutralising antibodies in the blood of pigs. Currently, however, there is no way to tell the difference between pigs which gained these antibodies from a vaccine, and those that were infected with the real virus.
“Unless a new diagnostic test is developed which can distinguish between infected and vaccinated animals, a NiV vaccine for pigs could not be used for routine vaccination, as it would interfere with surveillance for the virus,” explains Becca. Therefore, the team is now focused on designing this test.
If successful, the team can begin conversations with local governments and vaccine regulators about how the vaccine could be rolled out in pig populations. Ultimately, these efforts are an important part of the wider work of vaccinologists. Many other viruses in wild animal populations have the potential for zoonosis. We will not know which ones can successfully infect humans until it is too late, so the work of future generations of vaccinologists will be crucial to prepare for this eventuality.
PROFESSOR SIMON GRAHAM
The Pirbright Institute, UK
FIELD OF RESEARCH: Vaccinology
RESEARCH PROJECT: Developing a vaccine against Nipah virus in pigs
PROJECT PARTNERS: University of Oxford, UK; Australian Centre for Disease Preparedness, Australia; University of Queensland, Australia; International Centre for Diarrhoeal Disease Research, Bangladesh; Zoetis, USA
FUNDERS: This research is funded by the Department of Health and Social Care using UK Aid funding and is managed by Innovate UK. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health and Social Care.
Vaccinologists contribute to all steps of the vaccination process, from investigating the responses vaccinations trigger in the immune system, to developing the strategies and technologies required to deliver them in the real world. When West Africa was struck by an outbreak of Ebola virus in 2014, the world realised just how underprepared we were for large-scale outbreaks of zoonotic diseases.
Since then, the World Health Organization (WHO) has created a list of diseases that urgently require research and development of vaccines, and NiV has been identified as one of these priority diseases. “Because of these efforts, the scientific community was better prepared to respond to the COVID-19 pandemic,” says Simon. “Vaccines were developed against an entirely new virus in less than one year, which was a major achievement.”
WHY ARE VACCINES TESTED ON ANIMALS?
To ensure vaccines are safe for use, it is often crucial to study their effects on animals like pigs, whose immune systems behave in very similar ways to ours. “There are rightly lots of regulations and ethical considerations around the use of animal models,” says Becca. “We must use the fewest number of animals to get a significant result, and ensure we minimise any suffering.” Once a vaccine is proven to be safe, and that it enables the immune system to fight a virus effectively, it can then move on to human trials.
WHAT DOES A TYPICAL DAY OF WORK INVOLVE?
“Like all scientists, the life of a vaccinologist is always very varied,” says Simon. Some days will be spent at the desk, either planning new experiments, analysing data from past experiments, or getting up to speed with the latest research by reading academic journals. Other days will be spent in the lab, processing and analysing samples. No two days are ever the same!
WHY SHOULD YOU CONSIDER A CAREER IN VACCINOLOGY?
Vaccinology is a fast-moving field of science. It considers a huge range of diseases and organisms, and draws from many different fields of research. And there is still a lot of work to be done. New vaccine technologies are being developed constantly and there is a pressing need to prepare for viruses which we know are out there, but which have not yet been discovered. “This means there are pioneering discoveries occurring all the time, making it a very exciting area of research,” says Becca.
• As a vaccinologist, you could work for a pharmaceutical company or in a research institute. You might find yourself responsible for any or all stages of vaccine development, from microbiological investigation of pathogens, to designing and testing new vaccines, to getting a vaccine onto the market and into public use.
• The Pirbright Institute (www.pirbright.ac.uk), where Becca and Simon work, hosts many vaccinologists who are working to prevent and control viral diseases.
• The British Society for Immunology has a wealth of information about careers in the field (www.immunology.org/careers) and is running a campaign to celebrate vaccines (www.immunology.org/celebrate-vaccines).
• World-leading vaccinologist Professor Robin Shattock wrote an article titled ‘How to get a job in vaccine development’ for Nature: www.nature.com/articles/d41586-021-03057-6
• “Having a passion for science is a must,” says Becca. As it is a multidisciplinary science, there is no specific route to get into vaccinology, but you should be passionate about your chosen pathway.
• At school, biology, chemistry and maths will be useful subjects to study to enter relevant university degree programmes.
• Undergraduate degrees in biology, biochemistry, medicine, veterinary medicine or immunology could all lead to a career in vaccinology.
• Some pharmaceutical companies offer apprenticeships, allowing you to train as a vaccinologist while also working. In the UK, Pfizer supports the National Apprenticeship Programme: www.pfizer.co.uk/careers/apprenticeships
• Completing a PhD after university will allow you to follow an academic career path as a vaccinologist.
I have always had a passion for science and animals. When I was younger, I thought I wanted to be a vet, but I changed my mind after completing work experience. I knew I loved science and animals, so I decided to study a bio-veterinary science degree.
My PhD supervisors were huge inspirations to me. They taught me the foundations of my vaccinology knowledge, which I have been able to grow and develop in my post-doctoral job.
I love how varied each day of my job can be. I also really enjoy problem solving, which is something you have to do every day in science!
There are a number of challenges to overcome when developing vaccines. These include the initial design of the vaccine, testing the immune responses it creates, seeing whether it is protective and, finally, trying to get the vaccine onto the market. This relies on collaborating with lots of other scientists and regulatory bodies.
The highlight of my career has been getting involved in the COVID-19 response. I helped lead a team to assess the immune response to six different vaccine candidates in pigs, including the Oxford/AstraZeneca vaccine. My ambition for the future is to develop vaccines against other emerging infectious zoonotic diseases.
When I’m not working, I love spending time with my new little family. I have an 8-month-old son who is growing and changing all the time.
I have always had a love for animals – my earliest career ambition was to be a zookeeper! Over time, this passion evolved into science and my career in veterinary science marries these two interests.
I studied immunology and microbiology at university. I was fascinated by the ‘arms race’ that exists between the immune system and disease-causing microorganisms. But what really inspired me to pursue my career was the understanding of how vaccination can have such a major impact on controlling diseases.
Teamwork is what I enjoy most about my job. It involves working with lots of interesting people from different backgrounds. Science is a truly international business, and we collaborate with scientists in Europe, the Americas, Asia and Australia.
The challenges we face when developing new vaccines differ hugely, depending on the disease we are targeting. In some ways we were lucky with COVID-19, in that safe and effective vaccines were rapidly developed in such a short space of time. Compare that with HIV: after almost 40 years of research and billions of dollars spent, we still have no vaccine. But exciting progress is being made, and we are getting closer.
I spent six great years working in Nairobi, at the Kenyan International Livestock Research Institute. I worked as part of a team that made significant steps towards the development of a much needed next-generation vaccine for East Coast fever, a disease that kills at least one million cattle each year in Sub-Saharan Africa.
1. Keep persevering! There will be times when you feel a door has been slammed in your face, but remember, as one door closes, another opens.
2. To quote Einstein, “It’s not that I’m so smart, it’s just that I stay with problems longer”.
Write it in the comments box below and Becca or Simon will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)














