How can we discover new antibiotics?
First discovered in the 1920s, antibiotics have revolutionised the way we treat infectious disease. However, the discovery of new antibiotics has been declining since the mid-1960s and bacteria are gradually developing resistance to existing ones, posing an urgent threat to global health. At Princeton University in the US, Professor Mohammad Seyedsayamdost has developed a new method to identify novel natural products, potentially fast-tracking the development of new antibiotics
TALK LIKE A CHEMICAL MICROBIOLOGIST
ANTIBIOTICS – drugs used to treat infections by killing or preventing the growth of bacteria
ANTIBIOTIC RESISTANCE – the process of bacteria acquiring molecular mechanisms that protect from the effects of antibiotics
BACTERIA – single-celled microbes, some of which cause disease
BIOSYNTHESIS – the production of complex molecules within living organisms or cells
BIOSYNTHETIC GENE CLUSTERS – the sets of genes responsible for the production of natural products
CRYPTIC NATURAL PRODUCTS – natural products encoded by silent gene clusters
NATURAL PRODUCTS – small molecules produced by organisms to interact with and respond to their environment
SILENT GENE CLUSTERS – gene clusters that are inactive under standard laboratory conditions
Infectious diseases are one of the greatest threats to global health. The massive impact of the COVID-19 pandemic is a stark case in point. When it comes to treating bacterial infections, we rely on antibiotic drugs which kill or stop the growth of disease-causing bacteria. Alexander Fleming discovered the first natural antibiotic, penicillin, in 1928, sparking an age of antibiotic discovery which radically changed modern medicine. However, the development of antibiotics peaked in the early 1960s and has been steadily declining ever since. At the same time, cases of antibiotic resistance are rising around the world, as bacteria are evolving adaptations that increase their survival in the presence of antibiotics. Bacteria that are antibiotic resistant are becoming harder to treat, so the discovery of new antibiotics is more urgent than ever before.
To address this need, Professor Mohammad (Mo) Seyedsayamdost, a professor of chemistry and molecular biology at Princeton University, has been investigating important substances called ‘natural products’. “These are small organic molecules produced by living organisms that are released into the environment where they carry out a number of functions, including communication and competition with other organisms,” Mo explains. For example, natural products are used by bacteria to acquire nutrients, send signals to other microbes, and defend against competitors and predators in microbial warfare.
WHY ARE NATURAL PRODUCTS IMPORTANT?
For humans, natural products have provided a tremendous source of pharmaceutical drugs. “Over 70% of our current clinical antibiotics are based on this group of molecules as well as more than half of all Food and Drug Administration-approved drugs in the past 40 years,” says Mo. “Studying and discovering new natural products, therefore, can provide new drugs and therapeutically useful molecules.” Commonly used antibiotics like vancomycin and erythromycin are examples of how bacterial natural products have proved invaluable in a clinical setting.
However, the vast majority of natural products remain undiscovered. Bacteria have dedicated groups of genes called biosynthetic gene clusters, which are responsible for generating natural products. Recent research has revealed that only around 10% of these gene clusters are active under standard laboratory growth conditions, meaning there is an extensive reservoir of natural products that we have barely tapped into. These inactive gene clusters are described as ‘silent’. Since natural products are such an important source of drugs, finding ways to turn these silent gene clusters ‘on’ would have a profound impact on antibiotic discovery. This is exactly what Mo’s research group has succeeded in doing.
HOW IS MO INVESTIGATING NATURAL PRODUCTS?
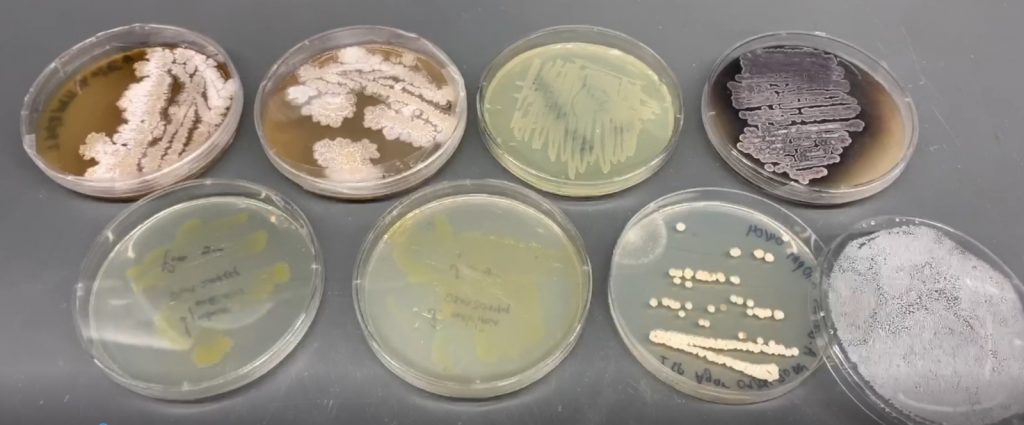
Usually, when bacteria are experimented with in a lab, they are grown in a nutrient-rich medium where there are no competitors or predators. However, this does not reflect the bacteria’s natural environment, which is much more complex and dynamic in terms of microbial life. “For example, one gram of soil, the source of many of the strains we study, harbours over 10,000 species of bacteria,” Mo explains. In the wild, bacteria are bombarded with signals and toxins from surrounding microbes, triggering them to make full use of the arsenal of natural products at their disposal so they can compete in this environment. In standard lab conditions, however, bacteria do not have the same incentive to produce as many natural products. “Behaviours that may occur naturally in a competitive, nutrient-limited context are therefore not replicated in the lab, which is why many genes stay silent in lab experiments,” says Mo.
Mo’s research team has developed a method called HiTES (High-Throughput Elicitor Screening) to trigger bacteria to produce more natural products in the lab. By exposing bacteria to molecules they would encounter in the wild, HiTES activates biosynthetic gene clusters to generate natural products that would otherwise be ‘cryptic’, or not produced, in a competitor-free environment.
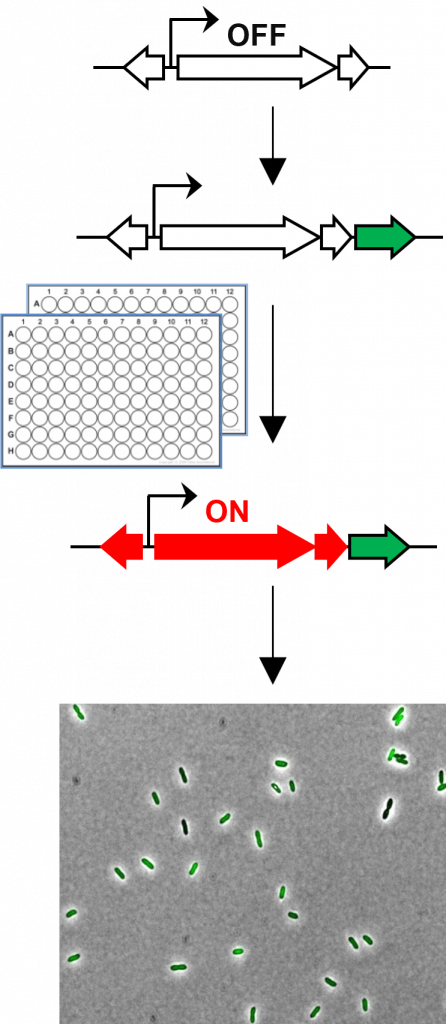
But how can they tell if a silent gene cluster has been activated? In one experiment, Mo tagged bacteria with a synthetic gene which caused the bacteria to turn fluorescent green when the silent gene cluster was activated. Then, the natural product produced by the activated gene cluster could be identified and isolated.
THE RESULTS SO FAR
Using HiTES, Mo’s team has accessed over 100 new, cryptic natural products. Some of these have proven more effective than current clinical antibiotics and so provide very promising drug leads for future antibiotic development. “One cryptic natural product that we have identified is 5-fold more potent than metronidazole, the current drug used against C. difficile infections,” says Mo. “Another proved 20-fold more potent than ribavirin, the standard medication used against RSV, which causes a type of respiratory infection.” Interestingly, Mo found that the molecules most effective at triggering new natural products from bacteria were current antibiotics when administered at low doses. This means that old antibiotics can be used to find new cryptic ones.
Reference
https://doi.org/10.33424/FUTURUM258
ANTIBIOTICS – drugs used to treat infections by killing or preventing the growth of bacteria
ANTIBIOTIC RESISTANCE – the process of bacteria acquiring molecular mechanisms that protect from the effects of antibiotics
BACTERIA – single-celled microbes, some of which cause disease
BIOSYNTHESIS – the production of complex molecules within living organisms or cells
BIOSYNTHETIC GENE CLUSTERS – the sets of genes responsible for the production of natural products
CRYPTIC NATURAL PRODUCTS – natural products encoded by silent gene clusters
NATURAL PRODUCTS – small molecules produced by organisms to interact with and respond to their environment
SILENT GENE CLUSTERS – gene clusters that are inactive under standard laboratory conditions
Infectious diseases are one of the greatest threats to global health. The massive impact of the COVID-19 pandemic is a stark case in point. When it comes to treating bacterial infections, we rely on antibiotic drugs which kill or stop the growth of disease-causing bacteria. Alexander Fleming discovered the first natural antibiotic, penicillin, in 1928, sparking an age of antibiotic discovery which radically changed modern medicine. However, the development of antibiotics peaked in the early 1960s and has been steadily declining ever since. At the same time, cases of antibiotic resistance are rising around the world, as bacteria are evolving adaptations that increase their survival in the presence of antibiotics. Bacteria that are antibiotic resistant are becoming harder to treat, so the discovery of new antibiotics is more urgent than ever before.
To address this need, Professor Mohammad (Mo) Seyedsayamdost, a professor of chemistry and molecular biology at Princeton University, has been investigating important substances called ‘natural products’. “These are small organic molecules produced by living organisms that are released into the environment where they carry out a number of functions, including communication and competition with other organisms,” Mo explains. For example, natural products are used by bacteria to acquire nutrients, send signals to other microbes, and defend against competitors and predators in microbial warfare.
WHY ARE NATURAL PRODUCTS IMPORTANT?
For humans, natural products have provided a tremendous source of pharmaceutical drugs. “Over 70% of our current clinical antibiotics are based on this group of molecules as well as more than half of all Food and Drug Administration-approved drugs in the past 40 years,” says Mo. “Studying and discovering new natural products, therefore, can provide new drugs and therapeutically useful molecules.” Commonly used antibiotics like vancomycin and erythromycin are examples of how bacterial natural products have proved invaluable in a clinical setting.
However, the vast majority of natural products remain undiscovered. Bacteria have dedicated groups of genes called biosynthetic gene clusters, which are responsible for generating natural products. Recent research has revealed that only around 10% of these gene clusters are active under standard laboratory growth conditions, meaning there is an extensive reservoir of natural products that we have barely tapped into. These inactive gene clusters are described as ‘silent’. Since natural products are such an important source of drugs, finding ways to turn these silent gene clusters ‘on’ would have a profound impact on antibiotic discovery. This is exactly what Mo’s research group has succeeded in doing.
HOW IS MO INVESTIGATING NATURAL PRODUCTS?
Usually, when bacteria are experimented with in a lab, they are grown in a nutrient-rich medium where there are no competitors or predators. However, this does not reflect the bacteria’s natural environment, which is much more complex and dynamic in terms of microbial life. “For example, one gram of soil, the source of many of the strains we study, harbours over 10,000 species of bacteria,” Mo explains. In the wild, bacteria are bombarded with signals and toxins from surrounding microbes, triggering them to make full use of the arsenal of natural products at their disposal so they can compete in this environment. In standard lab conditions, however, bacteria do not have the same incentive to produce as many natural products. “Behaviours that may occur naturally in a competitive, nutrient-limited context are therefore not replicated in the lab, which is why many genes stay silent in lab experiments,” says Mo.
Mo’s research team has developed a method called HiTES (High-Throughput Elicitor Screening) to trigger bacteria to produce more natural products in the lab. By exposing bacteria to molecules they would encounter in the wild, HiTES activates biosynthetic gene clusters to generate natural products that would otherwise be ‘cryptic’, or not produced, in a competitor-free environment.
But how can they tell if a silent gene cluster has been activated? In one experiment, Mo tagged bacteria with a synthetic gene which caused the bacteria to turn fluorescent green when the silent gene cluster was activated. Then, the natural product produced by the activated gene cluster could be identified and isolated.
THE RESULTS SO FAR
Using HiTES, Mo’s team has accessed over 100 new, cryptic natural products. Some of these have proven more effective than current clinical antibiotics and so provide very promising drug leads for future antibiotic development. “One cryptic natural product that we have identified is 5-fold more potent than metronidazole, the current drug used against C. difficile infections,” says Mo. “Another proved 20-fold more potent than ribavirin, the standard medication used against RSV, which causes a type of respiratory infection.” Interestingly, Mo found that the molecules most effective at triggering new natural products from bacteria were current antibiotics when administered at low doses. This means that old antibiotics can be used to find new cryptic ones.
These results set the stage for understanding how and why biosynthetic gene clusters are activated and opens the door to accessing a wealth of new bacterial natural products. Antibiotic resistance remains one of the greatest threats to global health, but this research and its potential to revive a new age of antibiotic discovery makes an important step towards combatting this crisis.
 PROFESSOR MOHAMMAD SEYEDSAYAMDOST
PROFESSOR MOHAMMAD SEYEDSAYAMDOST
Professor of Chemistry and Molecular Biology, Princeton University, USA
FIELD OF RESEARCH: Chemical Microbiology
RESEARCH PROJECT: Investigating bacterial natural products to discover novel antibiotics
FUNDERS: National Institutes of Health (NIH), Pew Biomedical Scholars Program, Searle Scholars Program, Burroughs Wellcome Fund
 PROFESSOR MOHAMMAD SEYEDSAYAMDOST
PROFESSOR MOHAMMAD SEYEDSAYAMDOST
Professor of Chemistry and Molecular Biology, Princeton University, USA
FIELD OF RESEARCH: Chemical Microbiology
RESEARCH PROJECT: Investigating bacterial natural products to discover novel antibiotics
FUNDERS: National Institutes of Health (NIH), Pew Biomedical Scholars Program, Searle Scholars Program, Burroughs Wellcome Fund
ABOUT CHEMICAL MICROBIOLOGY
Chemical microbiology is the study of the chemicals and chemical reactions involved in the biological processes that occur in microorganisms. In today’s global health climate, this field is more relevant than ever. Chemical microbiology has informed our ongoing efforts to combat COVID-19, providing scientists with a deeper understanding of viruses and the development of vaccines and antiviral drugs. While COVID-19 poses an immediate health challenge, the creeping threat of antibiotic resistance seriously endangers modern medicine and will have devastating consequences unless we find innovative solutions. Mo’s research into antibiotic natural products is a great illustration of how valuable chemical microbiology can be in tackling the problems of infectious diseases.
THE MICROBES WITHIN US
While drug development is a significant focus of chemical microbiology, the field has wide-ranging potential. Most microbes we come into contact with do not cause disease. For example, did you know that approximately 39 trillion microbes live inside the human body? The microbial communities inside us, primarily residing in the gut, are known as the human microbiome, which in recent years has emerged as a new frontier for understanding human health. The composition of our microbiomes has been linked to many conditions, from obesity to anxiety. Increasing our understanding of chemical reactions in the gut is just one other example of how chemical microbiology is being applied to develop novel therapeutic techniques.
THE IMPORTANCE OF INTERDISCIPLINARY RESEARCH
Chemical microbiology is a highly interdisciplinary research area, combining microbiology, genetics, biochemistry and organic chemistry (the branch of chemistry that deals with carbon-containing molecules). “Chemical microbiologists need to be generalists and specialists at the same time,” says Mo. Consider his work on natural products. This research requires expertise in the analysis of genomes and biosynthetic gene clusters, biochemical methods to isolate natural products and uncover biosynthetic pathways, and, finally, genetic and microbiological techniques for determining natural product functions. “In my mind, these various approaches go hand-in-hand as they reveal the full complexity, the different layers that underlie each single natural product,” says Mo.
WHERE COULD A CAREER IN CHEMICAL MICROBIOLOGY LEAD YOU?
It is common for chemical microbiologists to work in academia. You could work in or lead a university research lab, conducting scientific research and publishing your findings in academic journals. Chemical microbiologists also work for biotechnology and pharmaceutical companies, where the science of chemical microbiology is applied to the manufacturing of drugs. Working in industry may involve responsibilities such as data collection and analysis, managing lab operations and presenting findings to medical professionals.
EXPLORE A CAREER IN CHEMICAL MICROBIOLOGY
• Look out for relevant outreach schemes for young people in your area, such as talks or work experience opportunities. Mo’s department (chemistry.princeton.edu/diversity-inclusion/outreach) offers chemistry workshops, with experiments led by members of his research group, as well as the ‘Biochemistry Outreach Symposium’, an annual summer event targeted at high school students.
• Keep up to date with developments in the field by reading publications and attending events. Mo recommends the American Chemical Society (www.acs.org/content/acs/en.html) and American Society for Microbiology (www.asm.org) which offer great workshops, symposia and annual conferences.
• The ACS recently published an entire special issue (pubs.acs.org/toc/acbcct/15/5) dedicated to highlighting the latest discoveries in chemical microbiology. Have a read to explore the kind of research you could be doing as a chemical microbiologist.
• Mo recommends taking chemistry and biology at school, as research in chemical microbiology requires a solid foundation in both subjects.
• Higher education is necessary for a career in chemical microbiology. Universities generally do not offer undergraduate courses dedicated to chemical microbiology, so studying related fields such as microbiology, biochemistry, chemistry or biology are all possible routes. From there, you can specialise in chemical microbiology further down the line. Mo only narrowed down his field of study to the chemical microbiology of natural products after completing his PhD.
HOW DID MO BECOME A CHEMICAL MICROBIOLOGIST?
WHAT WERE YOUR INTERESTS WHEN YOU WERE YOUNGER?
I wanted to be a professional athlete when I was young. My parents had other ideas and gently nudged me onto the path that I’m on today. I entered college with a pre-medical track in mind, but then fell in love with organic chemistry and biochemistry.
WHO INSPIRED YOU TO BECOME A SCIENTIST?
The courses I took with Professor Phil Keehn (organic chemistry), Professor Melissa Moore (biochemistry) and Professor Liz Hedstrom (enzymology) were especially influential. I then joined Professor Hedstrom’s lab to conduct undergraduate research and fell in love with lab work and scientific research. My graduate and postdoctoral advisers (Professors JoAnne Stubbe, Jon Clardy and Roberto Kolter) were also fantastic mentors and role models and very supportive of my career.
HOW HAS YOUR CAREER PATHWAY LED YOU TO YOUR CURRENT RESEARCH?
During my undergraduate and graduate research, I focused on detailed mechanistic studies of important enzymes, which gave me a strong foundation in mechanistic biochemistry and enzymology. As a postdoc, I was introduced to the wonderful world of microbiology and natural products. In my independent career, I have combined these different approaches with the goal of obtaining a comprehensive, holistic understanding of microbial chemistry and natural products.
WHAT DO YOU ENJOY DOING OUTSIDE WORK?
Sports! Tennis, soccer and basketball – though there is not a whole lot of time outside of work.
MO’S TOP TIPS
01 Pursue your passion and carve your own path.
02 Embrace the unpredictability of research! Not knowing the answer going into a project and then finally uncovering it is what makes research exhilarating.
Do you have a question for Mo?
Write it in the comments box below and Mo will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)





Dear Professor,
My name is Muradharan Velappan from India, living in Chennai, basically I’m a Microbiologist, presently I am in final version of submission of my PhD thesis at AMET University Chennai,
Being an independent researcher I have pure love with discovering new antibiotic, it is very interesting your biography, further i need valuable feedback on identifying novel antibiotics that to in native plant metabolites. I understand that you are in busy, kindly reply, in free time.
Thank you
Sincerely
Muradharan Velappan
(PhD research scholar)
AMET University
Department of marine Biotechnology
Chennai, India
Hello,
Thank you for your interest. Please contact me at my Princeton email with any specific questions.
Best regards,
Mo.