How can we improve the cost-effectiveness of medical trials?
Before a new treatment can be given to patients, it must first undergo medical trials, to ensure it is safe and effective. Dr Howard Thom is a health economist at the University of Bristol, UK, who hopes to improve this time-consuming process by encouraging the use of ‘adaptive trials’. These trials could reduce unnecessary research, save time and money, lead to better decision making and, ultimately, improve patient care
GLOSSARY
ADAPTIVE TRIAL – a medical trial whose design is adapted as it progresses
VALUE OF INFORMATION – the economic value to a funder of conducting a piece of medical research, such as an RCT
HEALTH ECONOMICS – A multidisciplinary subject drawing on economics, statistics, sociology and other fields to assist healthcare decision makers in efficient and equitable resource allocation
COST-EFFECTIVENESS MODEL – A computer program using mathematics and statistics to estimate and compare the costs and effects of healthcare policy decisions
CONTROL ARM – the group of patients in a trial who receive standard of care (the currently available drug or surgical procedure)
INTERVENTION – any medical treatment being investigated by a trial
INTERVENTION ARM – the group of patients in a trial who receive the intervention under investigation
RANDOMISED CONTROLLED TRIAL (RCT) – a medical trial in which patients are randomly assigned to either a control or intervention arm
When did you last take some medication? Have you ever undergone surgery? We all rely on the medical profession at some point in our lives, but have you ever considered how medics and academic researchers know how to treat us and what drugs will help us most effectively? Before any treatment can be used on patients, it must be tested in medical trials. Randomised controlled trials (RCTs) are considered the gold standard for medical trials. If a new intervention (e.g. a drug or surgical procedure) is being tested, participating patients will be randomly assigned to either a ‘control’ arm, where they will receive the current standard of care or an ‘intervention’ arm, where they will receive the new intervention. By comparing the outcome of patients on each arm of an RCT, researchers can assess whether a new intervention is superior, or at least not inferior, to the current standard of care.
Recruiting patients for an RCT is an ongoing process as trials will require a high number of patients and it is practically impossible to recruit all of these at the beginning of the trial. Instead, recruitment and randomisation take place over several years. In conventional RCTs, all aspects of a trial (e.g. the recruitment process, the intervention) will remain the same over the period of the trial.
WHAT ARE ADAPTIVE RCTS?
Dr Howard Thom, a health economist at the University of Bristol, is investigating the advantages of adaptive RCTs over conventional RCTs. His work could help to reduce unnecessary research, save time and money, and help the National Health Service (NHS) make better decisions about patient care.
As the name suggests, adaptive medical trials can be adapted as the trial progresses. “Early results from a trial might show an intervention is underperforming, so is not effective, or is giving patients an unexpected side effect, so is not safe,” explains Howard. Adaptive trials can react accordingly to these early trial outcomes and drop arms of unsuccessful interventions. “Conversely, early results might show an intervention is outperforming all others, so the trial can be stopped early because the best intervention has been identified, or new arms can be added to test interventions that emerge during the trial.” In ‘adaptive enrichment trials’, the criteria for recruiting patients are adapted as the trial progresses, so the trial can focus on patients who will most benefit from the treatment.
WHAT ARE THE BENEFITS OF ADAPTIVE RCTS?
“The key advantage of adaptive RCTs is that patients avoid being randomised unnecessarily,” explains Howard. When a patient signs up to participate in an RCT, they may receive the latest therapeutic innovations if they are randomly assigned to an intervention arm, but there is always a risk that the new treatment does not work as well as existing treatments. Also, if they are randomly assigned to the control arm they will not personally benefit from being included in the trial.
In adaptive RCTs, fewer patients are randomised to underperforming interventions and only those patients expected to benefit the most are recruited, which saves time, money and effort, and maximises the potential benefits to patients. However, despite these advantages, adaptive medical trials are not widely adopted. Howard hopes to demonstrate the value of them to funders and patients.
THE COST-EFFECTIVENESS OF INTERVENTIONS
In the UK, the National Institute for Health and Care Excellence (NICE) evaluates the cost-effectiveness of healthcare interventions using health economic evaluation, by weighing up the costs of an intervention against its effects. “Costs can include the drug or healthcare technology itself, hospital admissions, treatments for side effects, and any visits to healthcare professionals,” says Howard. “Effects can be measured by the quantity of life years gained but also by the quality of those life years, with quality being affected by levels of pain, mobility, mental health and other factors.”
As the costs and effects of a new intervention are typically unknown, they must be modelled by health economics. Howard uses his skills in mathematics and computing to predict the costs and effects of healthcare interventions.
VALUE OF INFORMATION ANALYSIS
Howard conducts ‘value of information analysis’ to determine the cost-effectiveness of medical trials. He compares the conclusions of a cost-effectiveness model based on the information currently available with the information likely to be generated by a new adaptive trial. If the conclusions are predicted to change substantially, that adaptive trial has large value, and vice versa. This allows healthcare providers, such as the NHS, to determine the economic value of a new RCT. “If the value of a new trial is greater than the economic cost to run that trial, it is judged to be cost-effective.”
AN EXAMPLE OF AN ADAPTIVE TRIAL
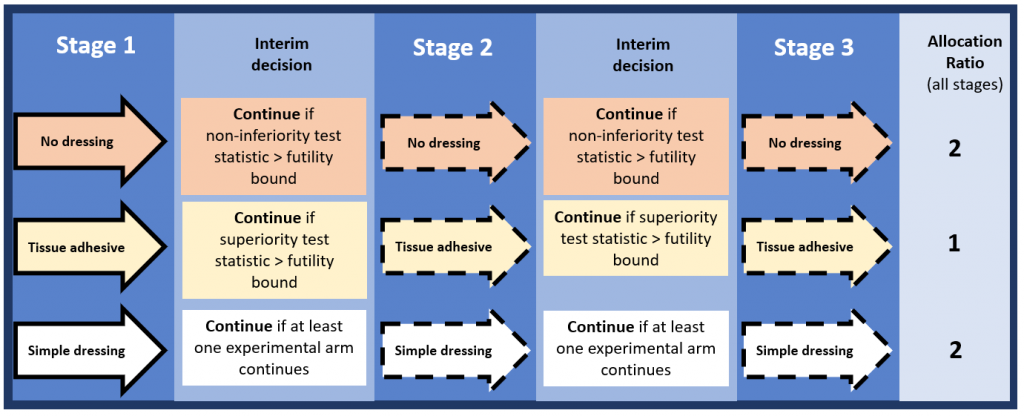
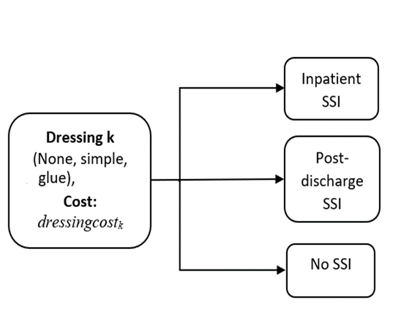
Howard has been estimating the value of conventional and adaptive RCTs in wound dressings for the prevention of surgical site infections. “Currently, there’s uncertainty as to whether simple dressings, tissue adhesive ‘glue’ dressings, or no dressings are the most cost-effective option for preventing infections after surgery, he explains. “Our proposed adaptive trial would begin randomising patients to all three options but would be able to drop arms or stop altogether based on two early-stage ‘interim’ analyses.” To test this with a conventional RCT would require over 25,000 patients, but an adaptive trial would need about 17,000 patients. Additionally, value of information analysis showed the value of the adaptive trial was equal to or greater than that of the conventional trial, indicating it was more cost-effective.
WHO IS INVOLVED?
Reference
https://doi.org/10.33424/FUTURUM233
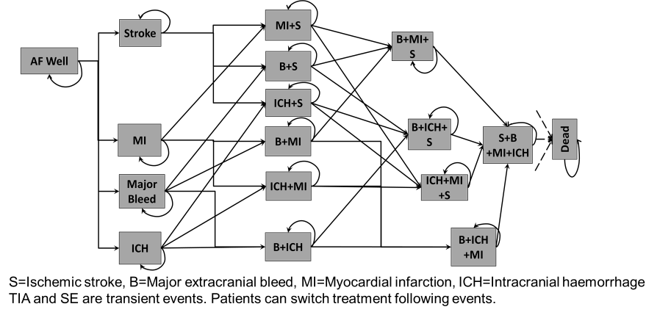
Howard and the team are using a Markov model to compare oral anticoagulants for the prevention of strokes in patients with atrial fibrillation. By using an adaptive trial to test different interventions, patients can switch between trail arms to help find the most effective treatment. www.bmj.com/content/359/bmj.j5058
Howard is using a cost-effectiveness model to determine the value of information for adaptive trial designs investigating the use of dressings to reduce surgical site infections (SSI). www.pubmed.ncbi.nlm.nih.gov/30556594
ADAPTIVE TRIAL – a medical trial whose design is adapted as it progresses
VALUE OF INFORMATION – the economic value to a funder of conducting a piece of medical research, such as an RCT
HEALTH ECONOMICS – A multidisciplinary subject drawing on economics, statistics, sociology and other fields to assist healthcare decision makers in efficient and equitable resource allocation
COST-EFFECTIVENESS MODEL – A computer program using mathematics and statistics to estimate and compare the costs and effects of healthcare policy decisions
CONTROL ARM – the group of patients in a trial who receive standard of care (the currently available drug or surgical procedure)
INTERVENTION – any medical treatment being investigated by a trial
INTERVENTION ARM – the group of patients in a trial who receive the intervention under investigation
RANDOMISED CONTROLLED TRIAL (RCT) – a medical trial in which patients are randomly assigned to either a control or intervention arm
When did you last take some medication? Have you ever undergone surgery? We all rely on the medical profession at some point in our lives, but have you ever considered how medics and academic researchers know how to treat us and what drugs will help us most effectively? Before any treatment can be used on patients, it must be tested in medical trials. Randomised controlled trials (RCTs) are considered the gold standard for medical trials. If a new intervention (e.g. a drug or surgical procedure) is being tested, participating patients will be randomly assigned to either a ‘control’ arm, where they will receive the current standard of care or an ‘intervention’ arm, where they will receive the new intervention. By comparing the outcome of patients on each arm of an RCT, researchers can assess whether a new intervention is superior, or at least not inferior, to the current standard of care.
Recruiting patients for an RCT is an ongoing process as trials will require a high number of patients and it is practically impossible to recruit all of these at the beginning of the trial. Instead, recruitment and randomisation take place over several years. In conventional RCTs, all aspects of a trial (e.g. the recruitment process, the intervention) will remain the same over the period of the trial.
WHAT ARE ADAPTIVE RCTS?
Dr Howard Thom, a health economist at the University of Bristol, is investigating the advantages of adaptive RCTs over conventional RCTs. His work could help to reduce unnecessary research, save time and money, and help the National Health Service (NHS) make better decisions about patient care.
As the name suggests, adaptive medical trials can be adapted as the trial progresses. “Early results from a trial might show an intervention is underperforming, so is not effective, or is giving patients an unexpected side effect, so is not safe,” explains Howard. Adaptive trials can react accordingly to these early trial outcomes and drop arms of unsuccessful interventions. “Conversely, early results might show an intervention is outperforming all others, so the trial can be stopped early because the best intervention has been identified, or new arms can be added to test interventions that emerge during the trial.” In ‘adaptive enrichment trials’, the criteria for recruiting patients are adapted as the trial progresses, so the trial can focus on patients who will most benefit from the treatment.
WHAT ARE THE BENEFITS OF ADAPTIVE RCTS?
“The key advantage of adaptive RCTs is that patients avoid being randomised unnecessarily,” explains Howard. When a patient signs up to participate in an RCT, they may receive the latest therapeutic innovations if they are randomly assigned to an intervention arm, but there is always a risk that the new treatment does not work as well as existing treatments. Also, if they are randomly assigned to the control arm they will not personally benefit from being included in the trial.
In adaptive RCTs, fewer patients are randomised to underperforming interventions and only those patients expected to benefit the most are recruited, which saves time, money and effort, and maximises the potential benefits to patients. However, despite these advantages, adaptive medical trials are not widely adopted. Howard hopes to demonstrate the value of them to funders and patients.
THE COST-EFFECTIVENESS OF INTERVENTIONS
In the UK, the National Institute for Health and Care Excellence (NICE) evaluates the cost-effectiveness of healthcare interventions using health economic evaluation, by weighing up the costs of an intervention against its effects. “Costs can include the drug or healthcare technology itself, hospital admissions, treatments for side effects, and any visits to healthcare professionals,” says Howard. “Effects can be measured by the quantity of life years gained but also by the quality of those life years, with quality being affected by levels of pain, mobility, mental health and other factors.”
As the costs and effects of a new intervention are typically unknown, they must be modelled by health economics. Howard uses his skills in mathematics and computing to predict the costs and effects of healthcare interventions.
VALUE OF INFORMATION ANALYSIS
Howard conducts ‘value of information analysis’ to determine the cost-effectiveness of medical trials. He compares the conclusions of a cost-effectiveness model based on the information currently available with the information likely to be generated by a new adaptive trial. If the conclusions are predicted to change substantially, that adaptive trial has large value, and vice versa. This allows healthcare providers, such as the NHS, to determine the economic value of a new RCT. “If the value of a new trial is greater than the economic cost to run that trial, it is judged to be cost-effective.”
AN EXAMPLE OF AN ADAPTIVE TRIAL
Howard has been estimating the value of conventional and adaptive RCTs in wound dressings for the prevention of surgical site infections. “Currently, there’s uncertainty as to whether simple dressings, tissue adhesive ‘glue’ dressings, or no dressings are the most cost-effective option for preventing infections after surgery, he explains. “Our proposed adaptive trial would begin randomising patients to all three options but would be able to drop arms or stop altogether based on two early-stage ‘interim’ analyses.” To test this with a conventional RCT would require over 25,000 patients, but an adaptive trial would need about 17,000 patients. Additionally, value of information analysis showed the value of the adaptive trial was equal to or greater than that of the conventional trial, indicating it was more cost-effective.
WHO IS INVOLVED?
At the University of Bristol, Howard works on a day-to-day basis with senior research associate Dr Mary Ward and receives advice from Professor Nicky Welton. All three are health economic modellers and experts in value of information analysis. They work with Professor James Wason and Dr Michael Grayling at Newcastle University, who are experts in adaptive trial designs. Additional input on scientific computing is provided by Dr Abdul-Lateef Haji-Ali at Heriot- Watt University in Scotland and, further afield, Dr Hawre Jalal, a health economist at the University of Ottawa in Canada. The application to surgical site infections was motivated by the Bluebelle study, led by Jane Blazeby, Professor of Surgery at University of Bristol. Professor Barnaby Reeves of the Bristol Trials Centre lead the RCT design for Bluebelle and advises Howard and his colleagues on practical aspects of RCTs on surgical site infections.
WHAT NEXT?
Howard and his team are now estimating the value of an RCT to assess oral anticoagulants used to prevent strokes during atrial fibrillation. There are several drugs in use for this, including coumarin, apixaban and dabigatran, but no RCT has ever directly compared them. Howard hopes an adaptive trial that drops arms early will require fewer patients, reach conclusions faster and be of greater economic value to research funders, while determining which treatment is most effective.
The team is also analysing an adaptive enrichment trial in surgical site infection. This trial will recruit patients with either ‘clean’ or ‘dirty’ wounds and as the trial progresses, recruitment will focus on specific patients with the wound type for which researchers are most uncertain of the optimal treatment, thereby maximising the trial’s value.
Beyond this, the team is interested in learning how patients view inclusion in adaptive trials. “It is very important (and in fact a legal obligation) that patients understand the design of trials they are agreeing to participate in, and this presents a challenge when trying to recruit people to more complex adaptive trials,” says Howard. “We are planning to work with patient representatives to learn the best way to explain adaptive trials and to understand concerns patients are likely to have about recruitment to such designs.”
 DR HOWARD THOM
DR HOWARD THOM
Bristol Medical School, University of Bristol, UK
FIELD OF RESEARCH: Health Economics
RESEARCH PROJECT: Investigating the advantages of adaptive trials over conventional randomised controlled trials when conducting medical trials for new treatments
FUNDERS: Medical Research Council (MRC), National Institute for Health Research (NIHR) Bristol Biomedical Research Centre (BRC)
 DR HOWARD THOM
DR HOWARD THOM
Bristol Medical School, University of Bristol, UK
FIELD OF RESEARCH: Health Economics
RESEARCH PROJECT: Investigating the advantages of adaptive trials over conventional randomised controlled trials when conducting medical trials for new treatments
FUNDERS: Medical Research Council (MRC), National Institute for Health Research (NIHR) Bristol Biomedical Research Centre (BRC)
ABOUT HEALTH ECONOMICS
Health economics is a diverse field but is generally centred on helping society decide how to spend money on healthcare in cost-effective ways. Such an undertaking requires a broad range of expertise, which is demonstrated by the breadth of research among Howard’s colleagues at the University of Bristol.
Dr Sabina Sanghera is studying how to measure and value quality of life in patients who have long-term conditions where their health is constantly changing, while Professor Joanna Coast has developed methods for measuring quality of life focused on the capabilities of patients rather than the severity of their symptoms. Dr Jo Thorn, Dr Sian Noble and Professor Will Hollingworth are using randomised controlled trials to investigate the cost-effectiveness of treatments.
“There is variety even within my own speciality of health economic modelling,” explains Howard. Professor Nicky Welton is using statistics to combine different types of evidence sources to decide whether new pharmaceuticals represent value for money, while Dr Elsa Marques is focused on economic modelling in orthopaedic surgery, looking at the cost-effectiveness of implants for hip and knee replacement.
“People working in health economics come from many disciplines,” says Howard, “including pharmacy, medicine, finance, mathematics, statistics, economics, computer science, anthropology and sociology.” Each person will bring their own unique set of skills and expertise, and this diverse range of backgrounds ensures the field of health economics is dynamic and relevant.
If you want to make a difference in people’s lives at the same time as doing something that you might not immediately associate with healthcare, then health economics might be the perfect arena for you!
EXPLORE A CAREER IN HEALTH ECONOMICS
• As a health economist, you could find yourself conducting research as part of a team like Health Economics Bristol at the University of Bristol (www.bristol.ac.uk/population-health-sciences/centres/healthecon) or for a think tank like the King’s Fund (www.kingsfund.org.uk), or working for a pharmaceutical company. You could advise the government about the economics of health policies or use your skills working for a regulatory agency such as the National Institute for Health and Care Excellence (www.nice.org.uk). Or, like Howard, you could provide commercial consulting services for companies such as Clifton Insight (www.cliftoninsight.co.uk).
• The International Society for Pharmacoeconomics and Outcomes Research (www.ispor.org/home) is the professional society for health economists. Howard recommends examining the titles and abstracts of upcoming conference presentations and workshops to see what is going on in the world of health economics.
• The Faculty of Health Sciences offers numerous opportunities for school students to experience life as a student at the University of Bristol (www.bristol.ac.uk/health-sciences/outreach), including summer schools and taster days.
• According to talent.com, the average health economist salary is £50,000, depending on position and experience: uk.talent.com/salary?job=health+economist are fun ways to network and build lateral skills.
PATHWAY FROM SCHOOL TO HEALTH ECONOMICS
• Because it is an interdisciplinary field, you can approach health economics from many different directions.
• At school, studying economics (if available) will be valuable. Business studies, geography and history will provide a strong background in the qualitative side of health economics, while maths and physics will prepare you for the quantitative side.
• At university, a number of degrees could lead you to the field. “I often work with people who came to health economics from sociology, economics, pharmacy and medicine,” says Howard. “To get into health economics modelling, good quantitative degrees, like mathematics, physics or computer science, are key.”
• Postgraduate degrees specialising in health economics are available at some universities, including the University of Bristol (www.bristol.ac.uk/study/postgraduate/2022/health-sciences/mschealth-economics-and-health-policyanalysis).
• This website lists some of the different routes you can take into the field of health economics: www.masterspublichealth.net/faq/how-do-you-become-a-healtheconomist
HOW DID HOWARD BECOME A HEALTH ECONOMIST?
WHAT WERE YOUR INTERESTS WHEN YOU WERE YOUNGER?
I was a massive nerd when I was young! I spent much of my free time programming simple computer games, studying maths and reading The Economist. At the time, I thought I’d become a games programmer or mathematician. I didn’t realise that the design of clinical trials required people with programming or maths skills.
HOW DID YOU BECOME A HEALTH ECONOMIST?
My undergraduate degree was in pure mathematics and, like many others who enjoyed maths but didn’t fancy a career as an impoverished number theorist, I was lured into doing a master’s in financial mathematics. I started this in 2008, but, during the opening week of my degree, the investment bank Lehman Brothers collapsed. This kicked off the global financial crisis and led me to re-evaluate my career prospects. Luckily, I’d completed a brief summer internship at the National Centre for Pharmacoeconomics in Ireland and had hugely enjoyed working on my first cost-effectiveness model, so I decided to complete a PhD in health economic modelling.
HOW DOES YOUR MATHEMATICAL BACKGROUND HELP YOU IN YOUR JOB?
My training in pure and financial mathematics is atypical of health economists, who usually come from an economics background, but is typical for health economic modellers. We need to be able to draw on diverse fields of mathematics, including probability theory, statistics, linear algebra and analysis, to build realistic models of healthcare interventions and patient outcomes.
WHAT ARE THE GREATEST JOYS OF BEING A HEALTH ECONOMIST?
I love solving a difficult health economic problem with some clever bit of maths or finding a new way to program a model that makes it faster or more realistic. These challenges are always new and exciting, and they’re often things that nobody else has done before. I also love having an impact on the world by improving healthcare delivery and research. The mathematical and programming challenges I solve lead directly to better evidence for decision makers, which leads to be better outcomes for patients.
WHY DID YOU ESTABLISH A HEALTH ECONOMICS CONSULTANCY COMPANY?
I founded Clifton Insight to allow me to work on further health economic problems. Pharmaceutical companies use health economics to demonstrate the value of their new interventions but have different data and challenges to governments and academics. For instance, they sponsor the RCTs themselves and they have access to individual patient data on which to base their models, which is rarely the case for academics. They also require much earlier stage modelling than in academic work, as it can begin before their interventions are licensed by regulators. These distinct types of challenges are what motivate me to do consulting work.
Write it in the comments box below and Howard will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)