How do enzymes speed up chemical reactions?
Enzymes are the catalysts of life. They accelerate chemical reactions inside cells to incredible speeds. Enzymes are so extraordinary that we extract them from organisms, like bacteria, and use them for countless processes, from cleaning our clothes and making cheese, to blood clot dissolution and improving antibiotics. Professor Judith Klinman at the University of California, Berkeley, USA, and her laboratory team, the Klinman Group, are focused on examining how enzymes generate these enormous rate accelerations.
TALK LIKE A BIOCHEMIST AND MOLECULAR BIOLOGIST
ACTIVE SITE — the area of an enzyme where a substrate molecule can attach
AMINO ACIDS — the building blocks which make up a protein molecule
CATALYSIS — a process of modifying the rate of a chemical reaction by adding a substance, called a catalyst, that is not consumed during the reaction
C-H CLEAVAGE REACTION — a reaction in which a carbon-hydrogen bond is broken and the hydrogen replaced with nitrogen, carbon or oxygen
COFACTORS — non-protein molecules that some enzymes require to function. These can be inorganic, such as a zinc ion, or organic, such as vitamins. Without cofactors, these enzymes would remain inactive
ENZYMES — most enzymes are a type of protein, which speed up (catalyse) biochemical reactions in plants, animals and microorganisms without being changed by the reaction
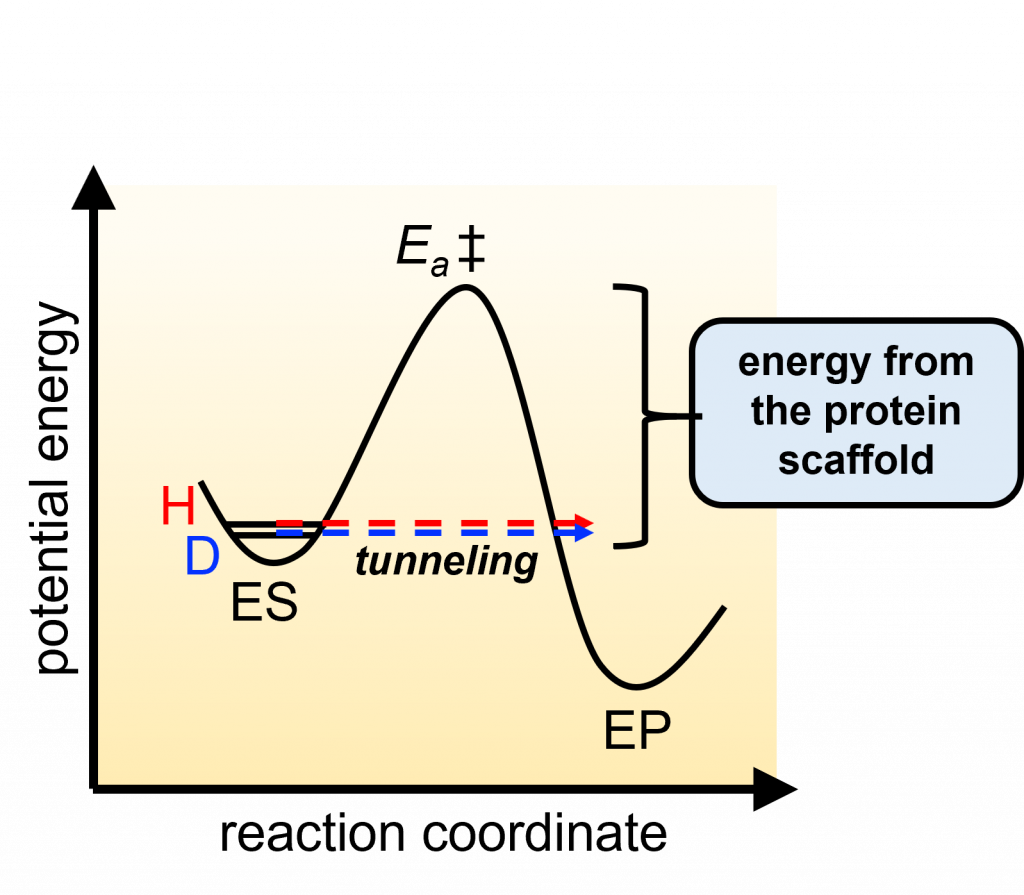
HYDROGEN TUNNELLING — the transfer of a hydrogen particle from a donor to an acceptor, where the hydrogen moves through a barrier rather than over the barrier
HEAVY METALS — are a group of metals that have high atomic weights or numbers and high densities, such as silver, zinc, copper, iron, lead, gold, platinum, mercury, cadmium and arsenic
ISOTOPIC — characteristic of two or more atoms that have the same atomic number (position in the periodic table) but different mass numbers
KINETIC (DEUTERIUM) ISOTOPE EFFECT (KIE) — an experiment in which an atom is replaced by its isotope and the reaction rate observed. Deuterium is an isotope of hydrogen, which is used in KIE experiments
PROTEINS — composed of chains of amino acids. Proteins have different three-dimensional shapes, which determine their function. There are four main categories of proteins in your body: structural, enzymes, hormones and antibodies
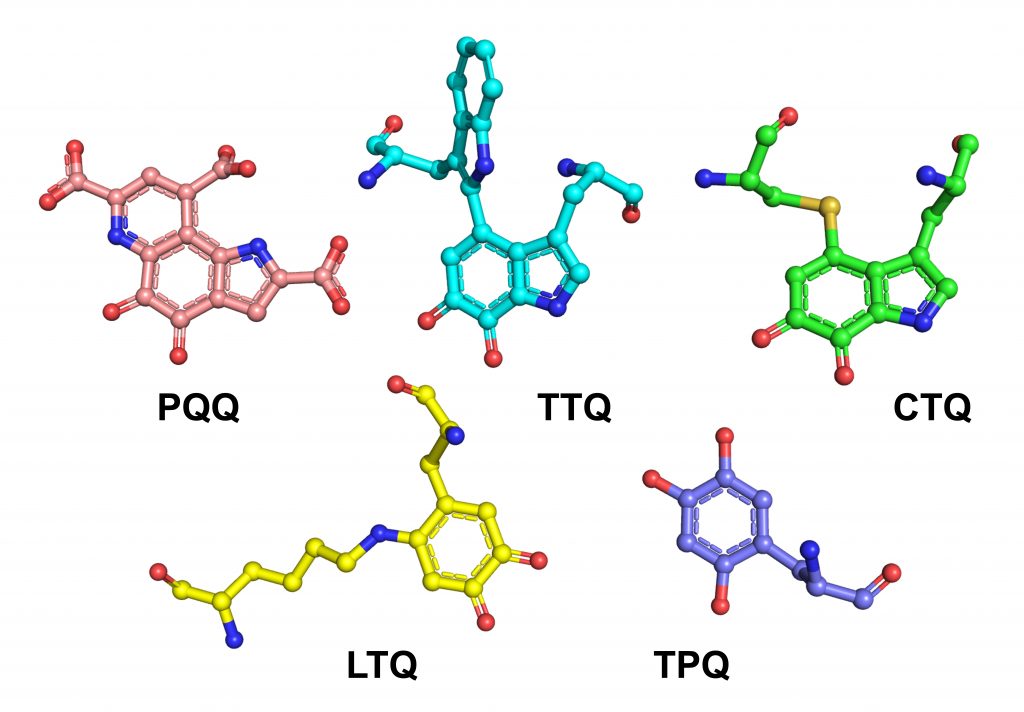
QUINO-COFACTOR — quinones that are generated from native amino acids within a folded enzyme’s active site and are essential for the enzymes’ catalysed reactions
REACTION RATE — the speed at which a chemical reaction occurs in relation to the concentration of the reactants
SPECIFICITY — the range within which a catalyst is active
SUBSTRATE — a substance on which enzymes act
THERMAL CONDUITS — materials that allow the transference of thermal energy/heat
Enzymes greatly enhance the rate of biochemical reactions in cells. Without them, we would not be able to exist. They are essential to life and also have extensive commercial uses, from making beer and cheese to the development of new drugs. Professor Judith Klinman is a Distinguished Professor of the Graduate School at the University of California, Berkeley. Focusing her research in biochemistry and molecular biology, she explores the factors that enable the astonishingly fast catalytic rates of enzymes.
ENZYMES AND HOW WE USE THEM
Thousands of chemical reactions happen in every cell in your body, every second, for your cells and body to function. Cells can die if these reactions occur too slowly, so they produce enzymes to increase the speed of these reactions at body temperature. They do this by binding their substrates, catalysing their chemical reactions and, finally, releasing the products. “Enzymes are the catalysts of life,” Judith explains. “They undertake all the chemical reactions inside the cell and do this really quickly: as much as 1030 times faster than the same reaction in the absence of the enzyme. That number, 1030 [times], is in the range of the number of stars in the universe!”
The catalytic properties of enzymes make them useful in a variety of industrial processes. Enzymes for commercial use are produced from the fermentation of specially selected strains of microorganisms or extracted from plant or animal sources. They can be used to catalyse a wide range of processes, from the modification of antibiotics to washing powders and cleaning agents; the possibilities are almost endless. “There are many people trying to create catalysts that are as good as the ones that have evolved over the billions of years of life on planet Earth,” Judith says. There is increasing demand for industrial processes that follow the principles of ‘green chemistry,’ where chemical products and processes that reduce or eliminate the use of hazardous substances are designed. Enzymes work under mild conditions of solvent, temperature and pressure, and can be used to undertake processes with less toxic effects on our environment.
THE KLINMAN GROUP
Despite more than half a century of detailed studies, there is still no satisfactory physical model for the enormous rate of accelerations generated by enzymes. “Our long-range goal has been to explore and examine the factors that enable the remarkably fast catalytic rates that enzymes have,” says Judith. The Klinman Group, Judith’s laboratory, has introduced and used many different kinds of approaches over the years to tackle this. One of the first approaches was the use of stable and radioactive isotopes that had begun to make it possible to follow the course of biological reactions and to identify reaction intermediates. The work of the Klinman Group is highly interdisciplinary, including physical, inorganic and organic chemistry, biochemistry, biophysics and molecular biology. “Our collaborations have been largely focused on the areas of X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, electron spin resonance (EPR) and electron-nuclear double resonance (ENDOR) spectroscopy and resonance Raman spectroscopy and theory,” explains Judith.
RESEARCH FINDINGS
Judith and her team discovered a new class of cofactors derived from the native amino acids of proteins, leading to novel quinone-based structures. “One of the really fascinating aspects of this work is the recognition that a single enzyme active site can first synthesize its new cofactor and then use it for the catalytic turnover of substrates,” says Judith.
Their work has also led to a unique opportunity to understand the contribution of protein dynamics to the specificity and reaction rates of enzyme catalysis. “In science, it is always important to be alert to observations that don’t comply with what we have assumed to be correct, and that is what happened while we were studying a number of different redox enzymes,” Judith explains. “Basically, the dominant mode for breaking a C-H bond involves moving over the top of a reaction barrier, while the data we were collecting showed that the transferred atom was moving through the barrier.”
Reference
https://doi.org/10.33424/FUTURUM308
She notes that “This phenomenon of ‘tunneling’ has been shown to be highly prevalent in biologically catalyzed C-H cleavage reactions. Hydrogen tunneling follows from the wave-particle duality of particles, with the wavelength of a particle being inversely related to its mass. To illustrate the point, I like to tell students that if they went on a (very serious) diet, they might get thin enough to be able to go out through the front door without ever getting up from the sofa!”
In the last few years, Judith and her team have uncovered a role for site-specific thermal conduits within each protein. These provide pathways for heat transfer from the solvent to the active site that act in synergy with the highly evolved properties of the active site.
The Klinman Group has further focused their research on reactions that are oxygen-dependent and played key roles as life transformed from initial anaerobic conditions to our current, O2-rich atmosphere. The team spent many years developing isotopic probes to study the mechanisms of oxygen activation by enzymes and to show how enzymes may prevent their own oxidation in this process. Several of the systems studied depend on the ions of heavy metals such as copper and iron.
 PROFESSOR JUDITH KLINMAN
PROFESSOR JUDITH KLINMAN
Distinguished Professor, Graduate School, University of California, Berkeley, USA
FIELDS OF RESEARCH: Biochemistry, Biophysics, Chemistry, Molecular Biology, Enzymology
RESEARCH PROJECT: Exploring the factors that enable enzymes’ remarkably fast catalytic rates
FUNDERS: The US National Institutes of Health (NIH) and the National Science Foundation (NSF)
ABOUT BIOCHEMISTRY AND MOLECULAR BIOLOGY
Molecular biology deals with the structure, properties and function of the basic molecular components of cells, from bacteria to plants and mammals. This greatly overlaps with biochemistry, which explores the chemical processes within and related to living organisms, focusing on processes at a molecular level. These fields affect many areas of science including biotechnology, medicine and agriculture. Research is helping to address global issues such as disease, food security and biotechnology.
WOMEN IN BIOSCIENCES
Presently, women are well represented in the molecular biosciences sector, but this was not always the case. Judith was the first female professor in any of the physical sciences at the University of California, Berkeley; the first tenured female professor in the chemistry department there and the first female chair of the chemistry department. “There really is an enormous difference between when I arrived on the Berkeley campus and now,” she explains. “Once women are readily accepted into programmes and see what is available to them, they just take off!”
PATHWAY FROM SCHOOL TO BIOCHEMISTRY AND MOLECULAR BIOLOGY
• Judith recommends having a solid background in biology, chemistry, physics and mathematics. Humanities are also important.
• Lab experience is very useful while at school and is essential in university where you can find a placement, internship or a year in an industry scheme.
• In the UK, you need A-Levels in sciences and maths to pursue an undergraduate degree in biochemistry or molecular biology. The exact A-Level requirements will depend on the course and university. For example, the BSc Biochemistry with Molecular Biology and Biotechnology at the University of Bristol, in the UK, requires three As at A-level, including chemistry and another core science or mathematics subject.
• Many people also advance to study for a postgraduate degree in the field.
EXPLORE CAREERS IN BIOCHEMISTRY AND MOLECULAR BIOLOGY
• Explore different paths and degree options for becoming a biochemist in the UK: nationalcareers.service.gov.uk/job-profiles/biochemist
• According to Career Explorer, the average salary for a biochemist in the US is $102,300: www.careerexplorer.com/careers/biochemist/salary/#how-much
• Biochemists can have careers in hospitals, universities, agriculture, food institutes, education, cosmetics, forensic crime research, and drug discovery and development.
• To get news and views on issues relevant to the molecular bioscience community, visit thebiochemistblog.com
• The Biomolecular Society promotes the advancement of biochemistry and molecular biology and has information on career paths, work experience, internships and more: www.biochemistry.org
• The Department of Chemistry at the University of California, Berkeley, has several outreach programmes: eduinfo.cchem.berkeley.edu/education-outreach.shtml
HOW DID JUDITH BECOME A BIOCHEMIST AND MOLECULAR BIOLOGIST?
Judith has a degree in chemistry and a PhD in physical organic chemistry, both from the University of Pennsylvania. She founded the Klinman Group and has received more than 21 honours and awards over the past three decades, including the National Medal of Science presented by former US President Barack Obama.
WHAT WERE YOUR INTERESTS WHEN YOU WERE GROWING UP?
From an early age, I was really interested in ballet. However, I realized at some point that I was not all that talented, though I still loved it! Growing up, I also loved the ocean and quiet, long walks on the beach, and I still have a strong affinity for nature. My mum directed me to books and art, while my stepdad was a resource once I got interested in science. It was two fantastic teachers from school who taught my physics and chemistry courses who ultimately inspired me to become a scientist.
HOW INTERDISCIPLINARY IS SCIENCE TODAY?
I think that once a scientist attempts to solve a major problem, the different disciplines come together easily. Science has become an increasingly interdisciplinary activity and, consequently, more collaborative. This has likely been accelerated by the introduction of the internet and much greater ease of communication. I believe that the humanities and science are not so far apart, and both serve the need in each of us to be creative.
WHAT INSPIRED THE CREATION OF THE KLINMAN GROUP?
I started out as a postdoctoral researcher and then a staff scientist at a research laboratory called ‘The Institute for Cancer Research’. As I began to learn the research tools in enzymology and read the literature, I became fascinated with the general field of enzyme catalysis. I was puzzled by the dominant theory for how enzymes give rise to their enormous rate accelerations, and this has guided much of my research efforts since towards a new view of the physical origins of enzyme catalysis.
WHAT HAVE BEEN THE EUREKA MOMENTS IN YOUR RESEARCH CAREER?
I am very proud of the achievements of the Klinman Group. One of our eureka moments was certainly when we figured out the first protein-derived, quino-cofactor structure. Finding enormous and unprecedented kinetic isotope effects that had never been validated before for reactions in condensed phase near room temperature was another eureka moment. Most recently, the uncovering of site-specific thermal conduits in enzymes that enable productive, long-range activation of distal enzyme active sites has changed our formalisms and created new avenues for research.
WHAT ARE YOUR AIMS FOR THE FUTURE?
I have just turned 81 years old and was planning on closing my laboratory before now. However, a number of new directions have emerged regarding (i) the role of solvent in initiating protein catalysis, (ii) the structural bases of protein thermal networks and whether these can be applied to new directions for catalyst design and regulation, (iii) whether we can find a satisfactory physical description for the transient initiation of extremely fast, long range and site-specific protein restructuring and, finally, (iv) whether it will be possible to observe the very rare events that actually lead to barrier crossings in enzyme processes. Clearly, these are big questions, for the next generation of young scientists. Still, we can’t resist trying to make some progress during the next few years!
HOW DO YOU TAKE TIME OFF FROM WORK?
I like to explore new books and authors, take long walks and hikes (especially by the ocean), work in the garden, hang out with my life partner and family and friends, and (since the COVID pandemic) ‘veg out’ in the evening with the latest series on TV. I also maintain a long-standing meditation practice that adds important balance to my life.
JUDITH’S TOP TIPS
01 Find an area where you are both strong academically and very interested – this is a dynamite combination!
02 Work with your teachers to learn how best to read the literature and to be especially attuned to finding something that interests you.
Do you have a question for Judith?
Write it in the comments box below and Judith will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)