How do nutrients and pollutants travel from rivers to the sea?
The Queen Mary University of London’s Professor Kate Spencer and Dr Jonathan Wheatland are on a quest to understand how suspended particulate matter carries vital nutrients and harmful pollutants from rivers to seas
TALK LIKE AN ENVIRONMENTAL SCIENTIST
Sediment – a naturally occurring material that settles to the bottom of a liquid
Pollutant – a substance that pollutes something and causes harm, especially to soil, water or the atmosphere
Pathogen – a bacterium, virus or other microorganism that can cause disease
Nutrient – a substance used by an organism to survive, grow and reproduce
Aggregate – a collection of particles that form a mass
Suspended particulate matter (SPM) – nanoscale to sand-size particles that are suspended in the water column of streams, rivers and lakes
Suspended particulate matter (SPM) is composed of incredibly small particles of solid matter that are suspended in the water. This matter can include fine grained mineral sediment, such as clay and silt, as well as organic matter from decaying plants and microbes. A high concentration of SPM is major cause of poor water quality and can affect aquatic wildlife.
The components that come together to create SPM are incredibly small. They generally measure between 10 and 1000 microns across (1000 microns is 1 millimetre). When they come together, they form loose, fragile aggregates that are sometimes called ‘flocs’. These aggregates are porous, which means they contain a lot of fluid-filled space.
Because SPM has a density that is similar to water, it can be easily suspended by water and travel immense distances. As it does, it carries important nutrients and sediments from rivers into the sea.
WHAT IS IT ABOUT SPM THAT IS OF INTEREST?
While the nutrients and sediments carried from rivers into the sea help sustain marine life, SPM can also contain pollutants, pathogens and even microplastics, which can be harmful. It is therefore important to understand more about SPM, including shape, size, structure and composition. Scientists want to know the mechanisms behind SPM to better understand the impact it has on ecosystems.
The Queen Mary University of London’s (QMUL’s) Professor Kate Spencer and Dr Jonathan Wheatland form part of a group that is attempting to develop our understanding of SPM. While many researchers have studied SPM, Kate and Jonathan have approached the subject from a unique perspective. “What is unique about our work is that we can capture these natural and complex aggregates and preserve their fragile structure,” explains Kate. “We can also look at scales from a few millimetres down to nanometres meaning we can examine everything from their overall size and shape, to the individual bacteria and clay minerals they contain.”
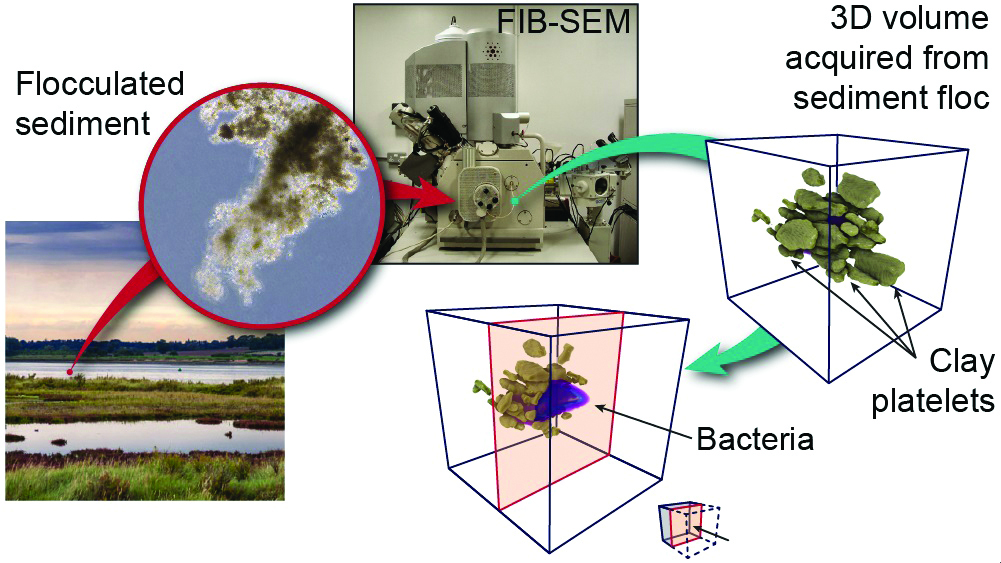
Using nano-tomography (a technique similar to CAT scans, but measuring much smaller samples), Kate and Jonathan can observe the internal structure of SPM in 3D and see how individual particles interact with each other. This image shows a collection of clay particles surrounding a single bacterium (pink). These particles are just a few microns in size. This image has been republished with approval from ACS: https://pubs.acs.org/doi/10.1021/acs.est.7b00770. Any requests to reuse this image should be directed to ACS: https://help.acs.org/
WHAT IS IMPORTANT ABOUT KATE AND JONATHAN’S RESEARCH?
The presence of SPM is essential to the healthy functioning of aquatic ecosystems. However, too much SPM can damage habitats and can lead to higher water treatment costs. Kate and Jonathan want to understand and predict how it moves through the water and where it finally settles. “Many water bodies are naturally very muddy. For example, estuaries contain large amounts of SPM, which is why our big rivers often look brown and dirty,” explains Kate. “However, poor land management practices, such as deforestation or the drainage of upland wetlands, can result in increased erosion on our hillslopes, delivering more particulate material to our rivers.”
The team wants to understand SPM’s composition and whether different types of pollutants or organic matter influence how it behaves in the water column. SPM has very complex shapes and structures, so sampling and analysing it can be very difficult. Other researchers have relied on techniques such as 2D cross sections, but these have produced a limited understanding. Kate and Jonathan are using techniques that will enable them to sample, observe and quantify the microstructure of SPM in 3D for the first time – this will help to develop our understanding of how SPM moves about and what it contains.
Reference
https://doi.org/10.33424/FUTURUM93
QMUL students in the Lake District in 2017.
Credit: Michelle Day
First-year BSc geographers, as part of the East Anglia field class, explored the long-term evolution of the coastline and the River Stour at Stutton Ness.
The river lab at QMUL’s School Geography.
© Joshua Ahmed
QMUL geography students on a field trip to the Lake District in the UK.
© Joana Costa Figueira
Sediment – a naturally occurring material that settles to the bottom of a liquid
Pollutant – a substance that pollutes something and causes harm, especially to soil, water or the atmosphere
Pathogen – a bacterium, virus or other microorganism that can cause disease
Nutrient – a substance used by an organism to survive, grow and reproduce
Aggregate – a collection of particles that form a mass
Suspended particulate matter (SPM) – nanoscale to sand-size particles that are suspended in the water column of streams, rivers and lakes
The components that come together to create SPM are incredibly small. They generally measure between 10 and 1000 microns across (1000 microns is 1 millimetre). When they come together, they form loose, fragile aggregates that are sometimes called ‘flocs’. These aggregates are porous, which means they contain a lot of fluid-filled space.
Because SPM has a density that is similar to water, it can be easily suspended by water and travel immense distances. As it does, it carries important nutrients and sediments from rivers into the sea.
WHAT IS IT ABOUT SPM THAT IS OF INTEREST?
While the nutrients and sediments carried from rivers into the sea help sustain marine life, SPM can also contain pollutants, pathogens and even microplastics, which can be harmful. It is therefore important to understand more about SPM, including shape, size, structure and composition. Scientists want to know the mechanisms behind SPM to better understand the impact it has on ecosystems.
The Queen Mary University of London’s (QMUL’s) Professor Kate Spencer and Dr Jonathan Wheatland form part of a group that is attempting to develop our understanding of SPM. While many researchers have studied SPM, Kate and Jonathan have approached the subject from a unique perspective. “What is unique about our work is that we can capture these natural and complex aggregates and preserve their fragile structure,” explains Kate. “We can also look at scales from a few millimetres down to nanometres meaning we can examine everything from their overall size and shape, to the individual bacteria and clay minerals they contain.”

Using nano-tomography (a technique similar to CAT scans, but measuring much smaller samples), Kate and Jonathan can observe the internal structure of SPM in 3D and see how individual particles interact with each other. This image shows a collection of clay particles surrounding a single bacterium (pink). These particles are just a few microns in size. This image has been republished with approval from ACS: https://pubs.acs.org/doi/10.1021/acs.est.7b00770. Any requests to reuse this image should be directed to ACS: https://help.acs.org/
WHAT IS IMPORTANT ABOUT KATE AND JONATHAN’S RESEARCH?
The presence of SPM is essential to the healthy functioning of aquatic ecosystems. However, too much SPM can damage habitats and can lead to higher water treatment costs. Kate and Jonathan want to understand and predict how it moves through the water and where it finally settles. “Many water bodies are naturally very muddy. For example, estuaries contain large amounts of SPM, which is why our big rivers often look brown and dirty,” explains Kate. “However, poor land management practices, such as deforestation or the drainage of upland wetlands, can result in increased erosion on our hillslopes, delivering more particulate material to our rivers.”
The team wants to understand SPM’s composition and whether different types of pollutants or organic matter influence how it behaves in the water column. SPM has very complex shapes and structures, so sampling and analysing it can be very difficult. Other researchers have relied on techniques such as 2D cross sections, but these have produced a limited understanding. Kate and Jonathan are using techniques that will enable them to sample, observe and quantify the microstructure of SPM in 3D for the first time – this will help to develop our understanding of how SPM moves about and what it contains.
WHERE DID THE IDEA COME FROM TO STUDY SPM USING SAMPLING TECHNIQUES FROM THE BIOMEDICAL SCIENCES?
Environmental and Earth scientists have been using CAT scans (computerised tomography) to look at the internal structure of sediments and soils for years. Kate, Jonathan and the team wanted to know if they could use other, similar techniques to look at much smaller samples and see the fine detail of SPM. So much of successful scientific research is based on collaboration and taking ideas from one field and transplanting them onto another.
“Our project is a collaboration between environmental scientists, materials scientists and engineers,” says Kate. “We worked closely with our colleagues in QMUL’s NanoVision Centre – a state-of-the-art microscope unit – to see what methods we could apply to our research.”
WHAT IS KATE’S TEAM HOPING TO ACHIEVE?
It is the first time that anybody anywhere has been able to observe the internal structure of SPM in 3D and see how individual particles interact with each other. Kate and Jonathan hope to use this information to improve their understanding of how SPM is transported. “We want to improve the mathematical models that predict how quickly SPM settles and erodes,” says Kate. “In the future, we also hope to use this information to understand how important SPM is for transporting pollutants and carbon through the aquatic environment.”
 KATE SPENCER
KATE SPENCER
Professor of Environmental Geochemistry
School of Geography
Queen Mary University of London, UK
DR JONATHAN WHEATLAND
Postdoctoral Research Assistant
School of Geography
Queen Mary University of London, UK
FIELD OF RESEARCH: Environmental Geochemistry
RESEARCH PROJECT: Kate and Jonathan are investigating suspended particulate matter (SPM) and observing it in 3D to see how individual particles interact with each other. Ultimately, they hope to determine the role SPM plays in transporting harmful materials into aquatic ecosystems.
FUNDER: UK Research and Innovation (UKRI)
MEET PROFESSOR KATE SPENCER AND DR JONATHAN WHEATLAND
WHAT DID YOU WANT TO BE WHEN YOU WERE YOUNGER?
KS: I never knew what I wanted to be, but I was always good at science and a very good communicator. I always liked nature and being outdoors – I cared a lot about protecting the environment, even when I was quite young. I did a degree in Earth Science that managed to combine all these skills and interests.
JW: I’ve always had an interest in understanding why things happen, particularly the processes that occur in the natural world. My fondest childhood memory is fossil hunting along the rugged coastline of Pembrokeshire in Wales – I’m sure at one point I wanted to be a palaeontologist! I ended up pursuing a degree in Geography and am glad I did because it has enabled me to work in some truly spectacular places.
WHO OR WHAT INSPIRED YOU TO BECOME A GEOCHEMIST?
KS: I didn’t know it existed as a subject until I was at university. In the final year there was a module called ‘Environmental Geochemistry’. I decided to take it and, as they say, the rest is history.
JW: I had very ‘outdoorsy’ holidays growing up and always wanted to do something related to the Earth Sciences.
KATE, YOUR BACKGROUND IS IN ENVIRONMENTAL GEOCHEMISTRY, BUT YOU ALSO WORK ON MUCH BROADER ENVIRONMENTAL PROJECTS LIKE THE ONE IN THIS ARTICLE. HOW ARE YOU ABLE TO DO THIS?
I’ve always had a very wide interest in coastal and aquatic environments, meaning that my research has been quite varied. My particular expertise is looking at the behaviour of pollutants and nutrients in the natural environment, but these skills can have many applications. I love working in a geography and environmental science department, because my colleagues and I are always looking for interdisciplinary approaches to understand and solve environmental problems.
IS THERE ANYTHING THAT PARTICULARLY WORRIES YOU ABOUT THE STATE OF OUR PLANET?
KS: I am concerned about how quickly and easily we create new chemicals and products with little understanding of how they behave or their potential impact. Microplastics are a hot topic in the news at the moment, but there are hundreds of new or ‘emerging’ chemicals that are developed for wide-ranging industrial, medical and even recreational purposes that end up in the environment. We can develop technologies to remediate and clean up the environment, but we all have a role to play as environmental citizens trying to consume and pollute less.
JW: I worry about how our society’s current attitude to the environment seems to be that it is an expendable and infinite resource. Modern life is often disconnected with nature and I think this has led to apathy with regards to its protection. That being said, I do believe that science can help solve the problems we have caused and, with the recent increase in ‘environmental awareness’, hopefully governments will begin to legislate ‘green’ policies.
WHAT INSPIRES YOU TO GET UP IN THE MORNING?
KS: Sunshine, conversation, having a long ‘to do’ list and a hungry cat!
JW: I’m not really a morning person, but if it’s sunny and I have a good idea I will often jump straight into my work – maybe after I have had one or two coffees!
IF YOU COULD GO BACK IN TIME AND START YOUR CAREER AGAIN, WHAT WOULD YOU DO DIFFERENTLY?
KS: Probably nothing. I didn’t have a plan at the start of my career, but I always felt that what I did was interesting and a lot of fun. I think I was good at spotting opportunities from the outset which has served me well.
JW: That’s a difficult question. There are a few things I might have done differently, but I am happy where I am with my career now. If I had to go back, I would perhaps try not to put too much pressure on myself (I am a bit of a worrier).
ABOUT ENVIRONMENTAL GEOCHEMISTRY
The negative impacts of human activity on the environment continue to be a talking point around the world. As such, the need for the environmental sector to try and ascertain the effects of particular actions is increasingly vital. This perhaps best explains why environmental geochemistry has grown rapidly over the past decade or so.
Environmental geochemistry is primarily concerned with understanding a whole manner of things associated with chemical elements and gases in the surface environment. Such environments might include bodies of water, crops, animals and humans. By understanding how such elements and gases come to be dispersed in such environments – and the effects of these dispersals – preventive measures can be put in place or motivations to act can be scientifically justified.
WHAT SORTS OF THINGS DO ENVIRONMENTAL GEOCHEMISTS STUDY?
“An environmental geochemist studies the abundance, distribution and behaviour of chemicals in the environment that are associated and sediment,” explains Kate. “The topic can be really broad but is extremely important for understanding everything from soil fertility to pollution.”
Clearly, there is no single thing that an environmental geochemist studies, but the breadth of topics – and the potential benefits – makes this an especially important field of enquiry, particularly in this day and age. Indeed, Kate’s background in environmental geochemistry has enabled her to work on much broader environmental projects such as the SPM imaging project described on pages 1 and 2.
WHAT ARE ENVIRONMENTAL GEOCHEMISTS TRYING TO ACHIEVE IN THEIR WORK?
As with many scientific fields, collaboration can be an essential mode of working, not least because one area of understanding can often be accentuated by combining with another. “Environmental geochemists often work closely with geologists, ecologists, hydrologists, soil scientists and geomorphologists,” says Kate. “Environmental geochemists are often trying to understand the impact that human activity has had on the environment, how important this is for ecological and human health, and how to remediate or solve these problems.”
Importantly, this is not a topic that is going to diminish in importance, so if you are interested in making a real difference in your career, environmental geochemistry is at the forefront of positive change.
ARE THERE MANY OPPORTUNITIES IN ENVIRONMENTAL GEOCHEMISTRY IN THE UK?
Environmental geochemistry is not a fleeting subject: it is a vital field of enquiry now, but one that will surely only increase in importance as time goes on. As such, whatever opportunities there are today, there are likely to be more tomorrow (metaphorically speaking). It is also worth bearing in mind that studying one subject, i.e. geochemistry, does not limit you to that one subject. Kate’s title may be Professor of Environmental Geochemistry, but she describes herself as an environmental scientist. Kate is based at QMUL’s School of Geography and her work is truly interdisciplinary, involving geography, geology and Earth science.
HOW TO BECOME AN ENVIRONMENTAL GEOCHEMIST AND ENVIRONMENTAL SCIENTIST
• The Royal Geographical Society has a Geography Ambassador scheme that recruits, trains and supports undergraduate geographers
• If you are interested in becoming a geochemist, Prospects has a dedicated section of its website which details all of the things you can expect from a career in geochemistry: https://www.prospects.ac.uk/job-profiles/geochemist
• According to The Geological Society, the starting salary for an environmental geochemist is anywhere between £20,000 and £30,000, with more senior positions earning between £32,000 and £50,000
PATHWAY FROM SCHOOL TO GEOCHEMIST/ ENVIRONMENTAL SCIENTIST
2 or 3 A-levels, or equivalent, including chemistry. Relevant degree areas include geography, Earth sciences, geosciences, physical, mathematical and applied sciences, and engineering
KATE’S TOP TIPS
1 It is important to gain a broad science background if you are going to embark on a career in geochemistry.
2 If possible, do your best to get an understanding of the natural environment as soon as possible. This will stand you in good stead when studying and it will aid you throughout your career.
JONATHAN’S TOP TIPS
1 Do something you love, no matter what career you end up doing. People forget that what they will be doing for their career will be for the majority of their life, so it is extremely important to prioritise your own happiness and wellbeing.
2 Remember that if you are interested in pursuing a career in science it can be tough, but the rewards you get out of it can be truly amazing. It is worth the hard work.
Do you have a question for Kate or Jonathan?
Write it in the comments box below and Kate or Jonathan will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)







