How do proteins affect heart health?
Proteins are the building blocks of life as they control the chemical reactions in cells. Despite the importance of proteins, scientists are still trying to determine what many of them do. One such scientist is Professor Beverly Rothermel, a molecular biologist at the University of Texas Southwestern Medical Center in the US, who has spent over twenty years investigating the many biological processes influenced by one particular protein, Regulator of Calcineurin 1.
TALK LIKE A MOLECULAR BIOLOGIST
Cardiac — relating to the heart
Chromosome — a molecule composed of DNA
Circadian rhythm — a process that naturally recurs on a 24-hour cycle
Degenerative — a progressive and irreversible deterioration
Down syndrome (DS) — a genetic disorder which causes developmental difficulties
Metabolically active — converting food into energy
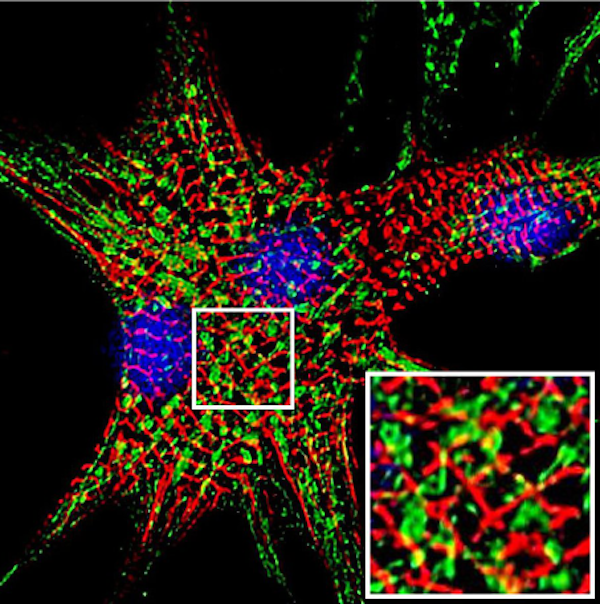
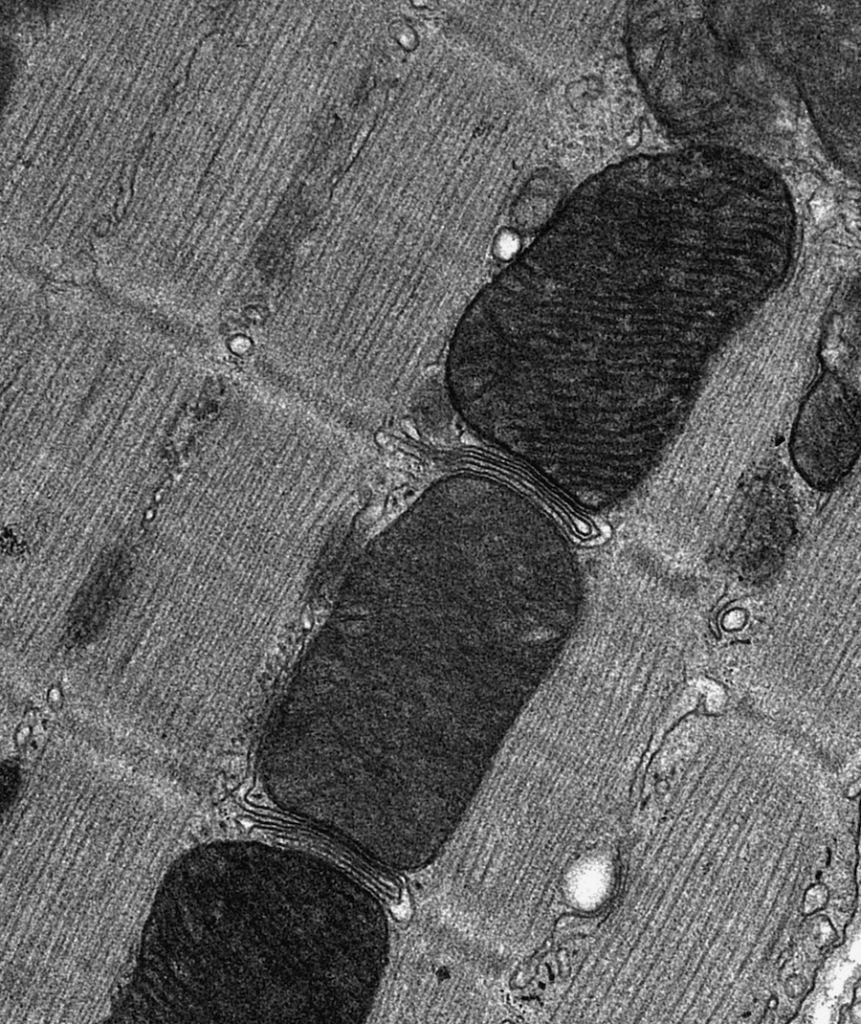
Mitochondria — the structures in cells that produce energy for the organism
Pathological remodelling — physical changes due to disease
Signalling — the process of a cell receiving, processing and emitting instructions
Calcineurin, also known as CaN, is a protein that is particularly important in the nervous, immune and cardiovascular systems. “CaN is estimated to make up more than 1% of the total protein weight of our brains, and yet we only know a fraction of what it does,” says Professor Beverly Rothermel, a molecular biologist at the University of Texas Southwestern Medical Center.
Bev’s research has been fundamental to understanding how Regulator of Calcineurin 1 (RCAN1) controls CaN activity and how this impacts human health. Her lab was involved in the initial discovery and characterisation of RCAN1, showing that when RCAN1 levels increase in a cell, the activity of CaN is reduced. This can have a significant effect because CaN is involved in many cellular processes. “The past twenty years of studying this remarkable protein have taken my laboratory in many interesting and often unexpected directions,” she says.
What role do CaN and RCAN1 play in the heart?
Heart disease is the number one cause of death worldwide. “The heart is very adaptable,” says Bev. “When you exercise, it undergoes beneficial remodelling to increase its power. In contrast, if it progresses to heart failure, it undergoes pathological remodelling. CaN signalling is known to play a prominent role in this process.” Bev and her team hypothesised that increasing RCAN1 levels might reduce the chance of heart disease.
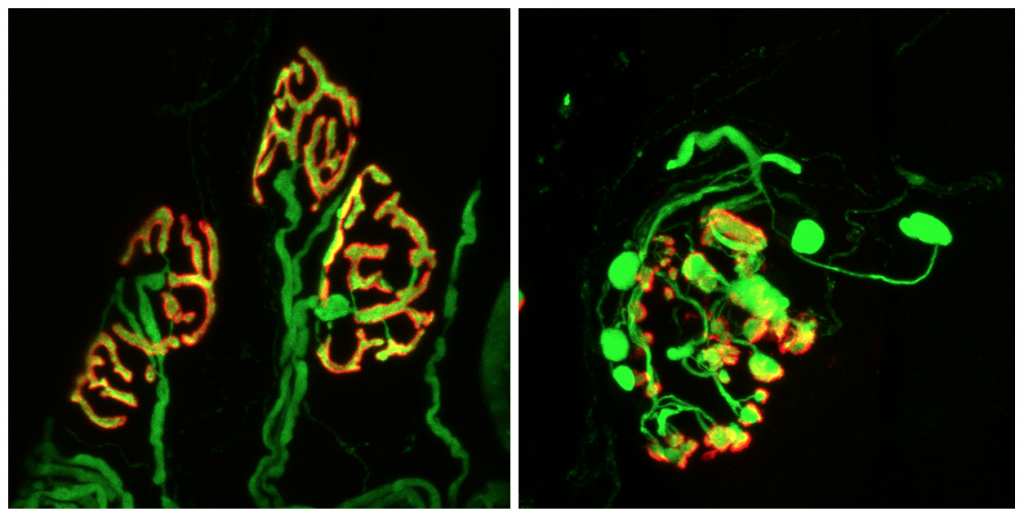
Bev used mice to test whether increasing levels of RCAN1 in the heart to inhibit CaN signalling would decrease the risk of heart failure. “We genetically engineered mice to have slightly higher levels of RCAN1 in the muscle cells of the heart,” she explains. Amazingly, the engineered mice had improved cardiac function in every model of heart failure that Bev and her team tested.
Where did this realisation lead Bev?
“Initially, we thought that whenever the heart is under stress, it increases levels of RCAN1 as a protective response,” Bev says. Although this is indeed true, Bev’s team accidentally discovered that hearts which were not subjected to stress also had huge daily fluctuations in RCAN1 levels. “In normal, healthy hearts, RCAN1 levels change as much as 20-fold over the course of a single day,” she says.
Since CaN levels are correlated with RCAN1 levels, this discovery suggested that CaN and RCAN1 are activated every day in a healthy heart, leading Bev and her team to the follow-on discovery that CaN and RCAN1 follow a circadian rhythm. “Many cardiovascular parameters are circadian in nature,” she says. “For instance, you are more likely to have a heart attack shortly after waking up than at any other time of day.”
By measuring RCAN1 levels in the hearts of her experimental mice at different times of day, Bev found RCAN1 levels were lowest when the mice were waking up and highest as they transitioned to sleep. Bev then simulated heart attacks in mice at different times of day, to test whether these changes in RCAN1 levels affect how much damage a heart attack will cause. She did this by first blocking the major blood vessel that provides blood to the heart (a process called ischemia) and then reopening the vessel to restore blood flow (a process called reperfusion). “This procedure is called ischemia/reperfusion, or I/R,” explains Bev. “It simulates what happens when a person has a heart attack (ischemia) and then makes it to hospital to have the blockage removed and blood flow to the heart restored (reperfusion).”
Bev and her team performed I/R on the mice around the time when they would normally wake up (and RCAN1 levels are low) and around the time they transitioned to rest (and RCAN1 levels are high). The results showed that there was almost twice as much damage to the heart muscle from I/R when it occurred just after the mouse woke up, compared to if the mouse was transitioning to rest, an observation also made in human heart attack patients. Using a drug to inhibit CaN was protective when I/R occurred at waking but provided no additional benefit at the transition to sleep when RCAN1 is present and the heart is inherently more protected.
How could this knowledge improve human heart health?
Bev’s findings are important because, while it is not possible to control when a person will have a heart attack, it is possible to carry out surgical procedures on the heart at optimal times. “Scheduling such procedures during times of day when the heart is more resistant to reperfusion damage could be a simple way to improve outcomes,” Bev explains. “Our studies are an excellent illustration of how the time of day that a treatment is administered can impact its efficacy.”
Most studies do not consider the impact that circadian rhythms may have on clinical outcomes. Bev speculates this may be a key reason why some treatments that seem very effective when tested on mice fail to work when tested on humans. “Mice are nocturnal, which means they sleep during the day,” explains Bev. “Most experiments are conducted during the day when mice should be sleeping, yet we try to apply these findings in humans when they are awake during the day.”
How do CaN and RCAN1 affect people with Down syndrome?
Another interest of Bev’s lab is the impact RCAN1 has on people with Down syndrome (DS). Humans typically have 23 pairs of chromosomes (46 chromosomes in total) which carry our genetic information. People with DS, however, have three copies of chromosome 21, instead of the usual two, meaning they have a total of 47 chromosomes. “This means people with DS carry three copies of any gene located on chromosome 21, which includes RCAN1,” says Bev.
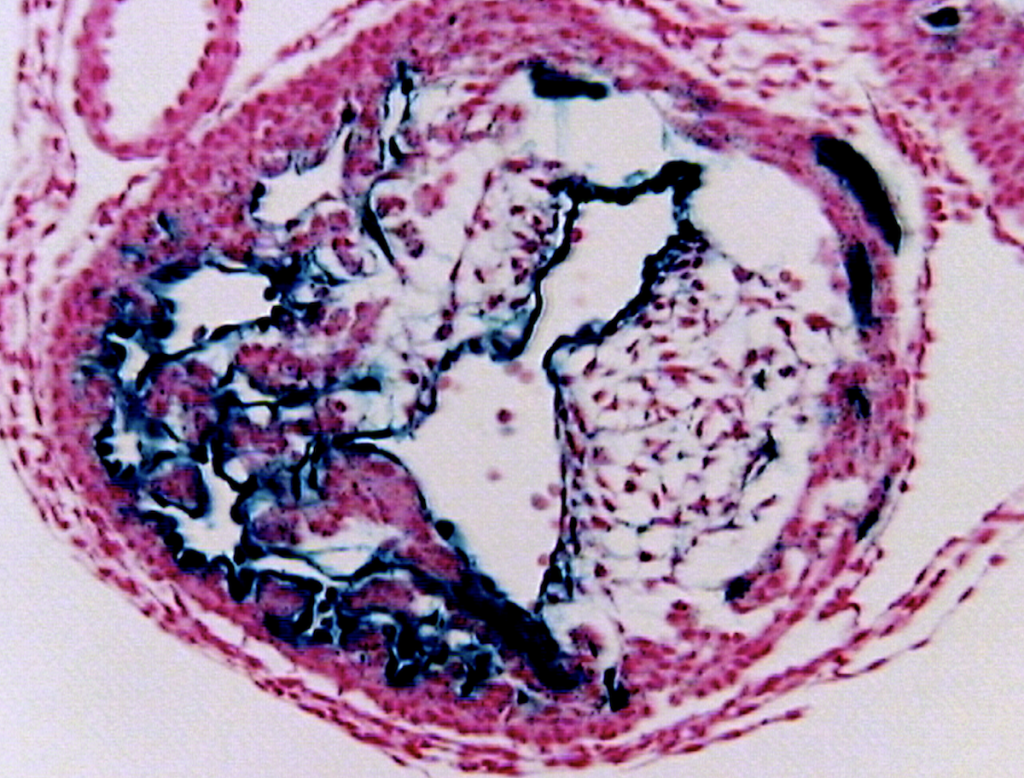
Bev and her team have been studying how this increase in RCAN1 impacts the mitochondria within the cells of people with DS. “Mitochondria are highly dynamic,” says Bev. “They are constantly combining with one another (fusion) or breaking apart (fission).” Mitochondria in a fused state are more metabolically active and produce more ATP (a molecule that provides energy), while the process of fission may reduce ATP production but allows damaged mitochondria to be removed and repaired, thereby keeping the mitochondrial population healthy. “Both fission and fusion are essential processes, and mutations disrupting either can cause degenerative disorders,” says Bev.
Reference
https://doi.org/10.33424/FUTURUM376
Other laboratories had shown that CaN activity promotes mitochondrial fission, so Bev hypothesised that an increase in RCAN1 in people with DS (due to the triplication of the gene on chromosome 21) may reduce mitochondrial fission, contributing to the degenerative nature of DS, as fused mitochondria are unable to repair themselves. By examining mitochondria in cells isolated from people with DS, Bev found evidence of increased fusion compared to in cells from people without DS. Reducing RCAN1 levels then restored a more normal mitochondrial behaviour.
Bev’s team is now genetically engineering mice with DS to explore how this may impact the health of humans with DS. “We already have some very exciting preliminary findings suggesting that increased RCAN1 levels and altered mitochondrial dynamics contribute to obesity in people with DS, particularly in females,” says Bev, highlighting how basic molecular biology research can yield new insights into critical health issues.
 Professor Beverly Rothermel
Professor Beverly Rothermel
Department of Internal Medicine, University of Texas Southwestern Medical Center, USA
Fields of research: Molecular Biology, Molecular Cardiology
Research project: Investigating the role of RCAN1 in cardiac health
Funders: National Institutes of Health (NIH; award R01 HD101006), The American Heart Association (AHA; award 19TPA34920001)
About Molecular Biology
Molecular biology is an interdisciplinary field combining biochemistry, microbiology, genetics and physiology. “A molecular cardiologist is a scientist who studies the heart at the molecular level, trying to understand how specific genes and proteins impact both normal cardiac function and the progression toward heart disease,” says Bev. Since heart disease is the cause of death for nearly 700,000 people in the US every year, working in this field can have a huge impact on improving lives.
“Modern science is a collaborative endeavour,” says Bev. Molecular biologists combine their knowledge with the expertise of other researchers to gain a better understanding of their work. “One of the things I enjoy about being a scientist is that you are always learning new things, synthesising new ideas and designing new experiments to test the validity of those ideas,” says Bev. “I’ve always loved figuring out puzzles. How wonderful to be able to make a living at it!”
What challenges will the next generation of molecular biologists address?
Bev believes the future of molecular biology will be shaped by two key challenges: the rapidly expanding wealth of new knowledge and the safe application of these findings to benefit human health. “Keeping up with the explosion of new data and techniques often feels a bit like trying to drink out of a firehose!” says Bev, as our understanding of cells increases with each new scientific contribution. “With the advent of genome-editing techniques, the field of molecular biology is entering a new era of possibility. The future depends on learning how to reap the benefits of these advances while anticipating and avoiding the pitfalls.”
Pathway from school to molecular biology
• “The best molecular biologists are integrative biologists, able to think and make hypotheses outside the confines of their own specific specialty,” says Bev. “Therefore, gaining a broad scientific foundation in physics, chemistry and mathematics is as important as learning about biochemistry and physiology.”
• At school, therefore, it would be good to study all science subjects in depth. Scientists need to explain their research to others, so language and writing classes are also useful.
• At university, study a bachelor’s degree such as biology, molecular biology, biophysics or biochemistry. If you want to work in research, you will then need to complete a master’s degree or PhD.
• To be successful as a molecular biologist, you will need strong critical thinking, analytical, communication and problem-solving skills.
Explore careers in molecular biology
• Molecular biologists may find themselves designing experiments to study biological tissues, using cloning or DNA sequencing techniques, analysing biological samples or studying genes.
• “The most useful thing you can do is seek out research opportunities in a lab as soon as possible, even if it is just washing dishes as a volunteer,” advises Bev. “Basic research is not for everyone, and the only way to know if it’s for you is to take part in it. Be careful though, it can be easy to get addicted!”
• Bev recommends taking your time to figure out what area you want to pursue. “The standard advice for being successful in science is to identify your field of interest as soon as possible and stay focused. I have violated this at multiple points, and although it may have slowed the pace of my career, I think I have become a more insightful scientist as a result.”
• The American Society for Biochemistry and Molecular Biology has a wealth of educational and career resources: www.asbmb.org/education/science-outreach
Meet Bev
I was very involved in music when I was younger. I played the cello and piano, sang, and studied music theory. Most people assumed I would pursue a career in music, but I had always planned on becoming a scientist. Both my parents were research chemists, and I always wanted to know what things were and how they worked.
When I started my undergraduate studies, my plan was to major in physics or biology, but definitely not chemistry. Then, I took my first biochemistry class, and I was hooked. This was only a few years after the first animal gene had been cloned into bacteria, and the tools to manipulate DNA were only just being developed. Who wouldn’t want to become a ‘Gene-Jockey’?
I completed a PhD at Yale and then two postdocs, but, though I was passionate about molecular biology, I was still trying to figure out where I fit in, as my work had covered plant genetics, mitochondria in yeast and cardiology. In the summer of 1989, shortly after CaN had been identified as a key player in heart disease, I heard a talk about a protein in yeast which inhibited CaN. This talk set my brain on fire, and I began to plan a series of experiments to test whether this protein also inhibited CaN in humans. I remember trying to explain to a non-scientist friend just how important I thought this gene was and that I might well be working on it for the rest of my career. Two months later, I had cloned RCAN1 from both human and mouse cells and shown that it inhibited CaN signalling in muscles.
In basic research, if 10% of your experiments are successful, then that’s a winning average on which you can build a career. In the first couple of years working on RCAN1, everything worked. While it was very gratifying, most of these initial experiments were the logical extension of things we already knew about CaN and muscle biology. I’m most proud of the unanticipated directions my lab has taken as we have pursued RCAN1, such as investigating circadian rhythms and mitochondrial dynamics.
Most summers, my lab hosts students who participate in research by testing one of ‘Bev’s crazy ideas’ to see if they might lead to productive research projects. Students get a feel for how basic research works by crafting a hypothesis, designing a controlled experiment and learning to discuss their findings in the context of a bigger picture. In return, the lab and I get to see things from a new perspective and find out whether we really understand what we’re doing.
Bev’s top tips
1. Ask questions and be sceptical. Don’t settle for a surface understanding of the things you learn.
2. Explore outside your comfort zone.
3. Don’t get discouraged when things aren’t working. Science, like most things of value, requires persistence.
Meet Rebecca

Rebecca Allen worked in Bev’s lab as a high school and undergraduate student. She is now about to qualify as a medical doctor and hopes to work as an emergency medicine physician.
When I was younger, I spent a lot of time waiting at the hospital for my aunt to finish her shifts as an intensive care nurse. Seeing patients in critical conditions, I was always mesmerised by the beeping and whooshing of the complicated machines that kept them alive. I knew I had to be part of that world. It was a place of wonder, curiosity and humanity.
Bev first invited me to her lab as a high school student when I was deciding if I wanted to pursue a career in medicine or research. I spent a summer cataloguing reagents and enzymes and conducting Western blot assays (preparing gels, mixing solutions, developing results, etc.). I then returned for two summers while in college, working with cells, flies and mice to contribute to Bev’s research on circadian rhythms.
There were countless benefits from working in Bev’s lab. I was exposed to brilliant women scientists, and it was inspiring to watch Bev lead her own lab team. It was incredible to witness the seemingly endless run of important experiments. I also had the opportunity to hear from, talk with and even shadow prominent scientists and medical professionals.
I come from a powerful and inspiring set of women healthcare professionals – many of my Filipino relatives are doctors and nurses in the US, and they have overcome great challenges. As one of very few women anaesthesiologists when she arrived in Texas, my aunt worked long hours and experienced micro- and macro-aggressions of every kind. While working as a nurse, my Godmother was once threatened by a doctor who said he would have her fired and deported. She is now pursuing her PhD in nursing, and it is her stethoscope I wear around my neck. It is an honour to wear the stethoscope she used to save so many lives and declare the end of many others. I am very proud that we will both graduate together, me with my medical degree and her with her PhD.
Rebecca’s top tips
1. Pick a mantra, write it on your mirror and read it aloud every time you wake up.
2. Seek research opportunities, whatever your age. Researchers are always eager to recruit young and upcoming scientists into their field.
Do you have a question for Bev?
Write it in the comments box below and Bev will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)