How to build a game-changing malaria vaccine
For millennia, malaria has plagued human civilization, killing billions over the course of history. Finding ways to combat this complex and clever disease remains a significant challenge. However, Professor Richard Bucala and his team at Yale School of Medicine in the US have found a way to develop a potentially super-effective vaccine – by turning the disease’s most devious tactics against it.
Talk like a malaria researcher
Antibody — a protein of the immune system that recognises a specific antigen
Antigen — a substance that induces an immune response in the body, in particular the production of antibodies
Biochip — in the context of this article, a portable biosensor able to analyse particular qualities of a sample of a substance
Cytokine — a type of protein produced by cells that modifies the immune response
Immune response — the response of the immune system to a harmful or foreign substance such as a pathogen
Immunological memory — the immune system’s ability to ‘remember’ past pathogens, so it can respond more rapidly and effectively when it encounters them again Inflammatory response — a response of the immune system that aims to mitigate the impact of harmful substances
Malaria — a disease caused by a mosquito-borne parasite that invades the body’s red blood cells
MIF (macrophage migration inhibitory factor) — an important cytokine that participates in inflammatory and immune responses
RNA (ribonucleic acid) — a nucleic acid, principally used in organisms to carry information from DNA. mRNA (messenger RNA) is a type of RNA found within cells that carries information from DNA in the nucleus to the cytoplasm, where it is used to make proteins. saRNA is an artificial form of RNA that contains genes that cause the saRNA molecule to be duplicated once inside cells
Parasite — an organism that lives in or on a different host organism and receives sustenance at the expense of the host RNA (ribonucleic acid) — a nucleic acid, principally used in organisms to carry information from DNA
Malaria is one of the world’s most deadly diseases, and despite gigantic investment in finding solutions, the unique nature of the disease continues to throw up obstacles. “It’s estimated that malaria has caused 50% of all human deaths since the Stone Age,” says Professor Richard (Rick) Bucala. “Even with today’s anti-malarial drugs and mosquito control strategies, malaria remains the world’s second leading cause of death by infectious disease.” Rick is a professor of medicine at the USA’s Yale School of Medicine and is busy unpicking malaria’s unique mechanisms to discover how to defeat it.
The development of malaria vaccines has not been straightforward. “The first malaria vaccine was approved for human use in 2015, although it provided protection for no more than half of individuals and its effectiveness waned significantly over time,” explains Rick. “In the latest clinical studies, a more recent vaccine has been shown to provide almost 80% protection.” Both vaccines work by teaching the body to identify a particular outer surface protein of the malaria parasite, but this protein varies between strains and is likely to mutate as malaria develops resistance to the vaccine. “There are significant costs involved in producing this protein-based vaccine, let alone the many variants that will need to be created, so it’s unclear what its global impact could be,” says Rick.
Our immune system
To understand why it is so difficult to develop a malaria vaccine first, we must first understand two important parts of our immune system, and the malaria parasite’s method for exploiting them. The first is the body’s ability to remember past pathogens and identify when the same pathogen is trying to invade the body again, allowing a rapid immune response before the pathogen has time to become established. It is this ‘immunological memory’ that forms the foundation of all vaccines, which typically involve providing the body with a pathogen identifier that the immune system remembers for future occasions.
The second involves some of the first molecules of an immune response, once a pathogen is identified. “Cytokines are the hormones of our immune system and regulate the strength and direction of our inflammatory and immune responses to an invading pathogen,” says Rick. “One such cytokine, MIF (macrophage migration inhibitory factor), is one of the very first immune mediators to be released and acts broadly to ensure strong inflammatory responses.” As it turns out, MIF is at the heart of the malaria puzzle – but not in the way that might be expected.
The MIF Mystery
Malaria escapes immunological memory, making it a real challenge for the body to develop an effective immune response to re-infection. It is for this reason that the disease has existed for so long, as human populations do not develop immunological resistance to it. It also explains why developing vaccines has been such a challenge. “It has long been known that individuals infected with malaria do not mount protective immune responses and always remain susceptible to re-infection,” says Rick. “Moreover, current candidate vaccines for malaria don’t elicit an effective immunological memory response.”
Reference
https://doi.org/10.33424/FUTURUM362
The mechanisms by which malaria avoided immunological memory remained a mystery for many decades. The plot thickened when, in the early 2000s, research found that malaria parasites have their own gene for the cytokine MIF, meaning they produce a version of MIF themselves when infecting a host. “Given MIF’s important role in the immune system, the finding that these parasites produce their own inflammatory cytokine was extremely puzzling,” says Rick. “A talented student in my lab, Tiffany Sun, decided to investigate this puzzle for her PhD thesis.” Tiffany studied a strain of malaria engineered with the MIF gene removed and used it to infect mice. These mice had a much more effective immune response and immunological memory than mice infected with a malaria strain that had its MIF gene.
These results indicated that the malaria’s MIF gene must be behind the suppression of the body’s immunological memory. “Further experiments showed that the strong inflammatory response caused by the parasite MIF gene led to the death of the particular immune cells responsible for memory development,” says Rick. “The parasite is essentially exploiting the body’s own inflammatory response through the use of MIF.”
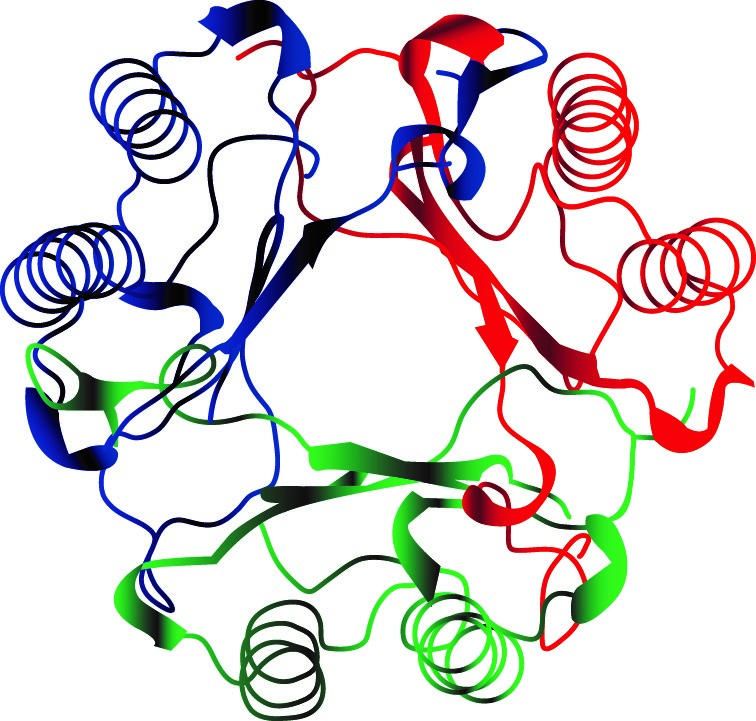
The MIF molecule, with each colour showing one submit of the trimeric protein structure.
A vaccine Holy Grail?
Rick’s lab was excited by these results, as it potentially indicated a chink in the malaria parasite’s armour. In 2011, Rick and his team got to work developing a vaccine, not based on proteins as with traditional vaccines, but rather using newly developed techniques based on RNA. RNA is a nucleic acid, like DNA, and is used within cells as the ‘code’ to produce proteins. Typical vaccines involve the injection of antigens – proteins present on pathogens that are identified by specific antibodies. Instead, RNA vaccines involve the injection of antigen-coding RNA into a body’s cells to stimulate them to produce these antigens themselves, essentially kickstarting immunological memory without exposure to the disease itself. Most RNA vaccines of this type use the most ‘standard’ form of RNA, known as messenger RNA (mRNA). “mRNA technologies have received a lot of attention because of the success of the COVID-19 vaccines, which were the first mRNA-based vaccines to be developed,” explains Rick.
Rick’s lab is using an engineered RNA type called self-amplifying RNA (saRNA). “saRNA contains genes that allow the RNA to replicate once it reaches the inside of cells,” says Rick. “This means much less RNA is needed for vaccination compared to mRNA vaccines, significantly reducing the cost of production.” This is an important feature for a malaria vaccine, given it is most prevalent in less developed nations where resources are scarce.
The choice of antigen is critical to an effective vaccine, and it is at this point that the lab’s work on parasite MIF comes in. The antigen Rick and his team are stimulating the body to produce and learn to recognise is none other than parasite MIF. “We are targeting the precise mechanism the parasite uses to evade the development of a protective immune response,” says Rick. “Our studies with animal models show that once this mechanism is neutralised, the infected host mounts a fully protective and long-lasting immune response that both eliminates the parasite and prevents re-infection.”
The vaccine Rick and his team are developing also has one extra important asset compared to other malaria vaccines: longevity. “Because parasite MIF must be able to mimic our own MIF to stimulate our immune system, it can’t mutate to evade detection like other antigens can,” says Rick. “While other candidate malaria vaccines use structural antigens which can vary between malaria strains and mutate over time, the parasite MIF antigen is highly conserved – meaning the development of malaria strains resistant to our vaccine would be exceedingly unlikely if not impossible.” If this vaccine can be approved for human use and scaled up, it might just provide the global battle against malaria with the silver bullet that has been sought for so long.
 Professor Richard (Rick) Bucala
Professor Richard (Rick) Bucala
Professor of Medicine, Pathology and Epidemiology & Public Health, Yale School of Medicine, USA
Fields of research: Immunology, Autoimmunity, Infectious Diseases, Vaccine and Drug Development
Research project: Developing an effective scalable saRNA vaccine against malaria that targets parasite MIF
Funders: National Institutes of Health (NIH), Mathers Foundation, Open Philanthropy
ABOUT MALARIA RESEARCH
Combatting malaria is one of humanity’s most significant challenges, with huge potential to save lives and prevent suffering. Rick explains more about his own research and the wider battle against malaria.
“The global problem of malaria is one of humanity’s greatest unmet medical needs. It kills around 600,000 people annually, mostly children, and has a huge societal and economic cost to many countries, too. This makes research into malaria important and potentially incredibly impactful. “Perhaps the most frustrating aspect of this work is that malaria is preventable. It is a tragedy that it occurs in those countries with the fewest resources to prevent or manage its societal cost. Resistance to antibiotic treatment is developing and prevention strategies such as mosquito nets (that can, for example, go around beds to prevent malaria-carrying mosquitoes getting in) are only partially effective. Having an effective, low-cost vaccine would be a vital new tool towards controlling and even eliminating malaria.
“My lab is multidisciplinary, and this is important for research into malaria vaccines. My own graduate training was in biochemistry and immunology. Knowledge of protein biochemistry is essential for the design of vaccine antigens, and knowledge of molecular biology and RNA is necessary for producing the constituents of RNA vaccines. Having experience with immunology and infectious disease models in mice also is essential.
“Some years ago, my lab developed a biochip that allows the direct visual detection of a person’s MIF alleles (different versions of a gene). Curiously, alleles that lead to low expression of MIF are especially common in sub-Saharan African populations, where malaria is prevalent. We reasoned that because lethal malaria results from excessive inflammation, which MIF helps to drive, it makes sense that low-expression MIF alleles are found in populations historically exposed to malaria. It suggests infected individuals have less excessive inflammation and so are more likely to survive. Our biochips helped us demonstrate this, showing that children with low-expression MIF alleles, who were infected with malaria, were less likely to have severe manifestations of the disease.
“The importance of malaria research has been recognised for over a century. In 1902, a British physician named Sir Ronald Ross received the Nobel Prize in Medicine for demonstrating the mosquito transmission of malaria parasites. In 2015, Tu Youyou shared the Nobel Prize in Medicine for her discovery of artemisinin, currently the most effective treatment for malaria. She is the first person from mainland China to receive a Nobel Prize in science, and did so without a doctorate or advanced medical degree.”
Pathway from school to malaria research
As Rick says, interdisciplinarity is essential for effective malaria research, which means the topic can be approached from a wide variety of educational backgrounds. To study biochemistry or immunology, like Rick did, universities typically want to see high school and college qualifications like biology, chemistry and mathematics. Depending on the course, other useful subjects can include further sciences, like physics and psychology, geography and the arts.
Explore careers in malaria research
• Rick says that the Yale School of Medicine has programmes to sponsor high school students in laboratories, with participation of Yale faculty scientists: medicine.yale.edu/edu
• There are opportunities available at many scientific institutions for high school students to get involved with research. For instance, the National Institute of Allergy and Infectious Diseases (NIAID) runs a summer internship programme for high school students at its laboratories in Maryland and Montana, in the US, with opportunities to conduct research into infectious diseases. Find out more: www.niaid.nih.gov/about/summer-internship-program#HS-SIP
• Jobs within medicine research vary widely in both scope and salary. According to salary.com, the average salary for a medical research scientist in the USA is around $93,000.
Meet Rick
When I was younger, I was inspired by the elegance and beauty of molecular structures and their capacity to explain how biology works. Later, I was motivated by the stories of scientists who designed and created new molecules to treat disease and improve health. I was especially inspired by the career of Louis Pasteur, who made key scientific insights by investigating the practical needs of his time, including the germ theory of disease, fermentation, vaccination and the chemistry of isomerisation.
My greatest ambition in medicine is to find new ways to treat disease. I felt that a career in medical research, rather than becoming a health practitioner, was the best way to achieve this goal. Initially, I embarked on the study of medicine because I thought it would make me a better scientist. Once I began to take care of patients, however, I saw how much physicians can help patients through the simple power of knowledge and reasoning. I also began to appreciate the complexity of medicine, and how so many factors, including social and humanistic factors, have to be considered to arrive at the best treatments and, ultimately, the best research questions.
I have trained over 60 scientists during my career. Trainees often bring a fresh and, I would say, fervent approach to scientific questions, using their individual perspectives and educational backgrounds to provide unexpected insights, which often leads to novel and creative solutions. It’s always stimulating to work with younger investigators and see them go on to tackle new areas of research with confidence and independence.
Rick’s top tip
The public health importance of combatting malaria is an enduring source of inspiration. Because the problem is so multifaceted, involving epidemiology, mosquito ecology, parasite biology, immunology and genetics, the opportunity to contribute, collaborate and learn across disciplines can be incredibly rewarding. Ask yourself how you could contribute – be inspired!
Do you have a question for Rick?
Write it in the comments box below and Rick will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)