Investigating the root of inflammatory diseases
Inflammation is an essential part of our immune system, but when it goes wrong, it can lead to serious diseases such as lupus and psoriasis. Professor Hans Haecker and his team at the University of Utah in the US are investigating the molecular components of cells that drive our inflammatory response, and screening for molecules that may help treat chronic inflammatory diseases.
TALK LIKE A MICROBIOLOGIST AND IMMUNOLOGIST
Autoimmune disease — when the body’s immune system attacks the body’s own cells by mistake
Immune system — all the parts of the body (including white blood cells and the lymphatic system) that help it fight infections and other diseases
Inflammation — when the body’s white blood cells produce chemicals that stimulate aspects of the immune system, with physical symptoms including redness and swelling
Intracellular — located or occurring within a cell or cells
In vitro — a term for experiments that take place outside of a living organism
In vivo — a term for experiments that take place inside of a living organism
Lupus — a chronic inflammatory disease with symptoms including joint pain, skin rashes and organ damage, in particular kidney injury
Phenotypic screening — a drug discovery strategy to identify molecules that affect a cell’s characteristics
Progenitor cells — stem cell descendants with the limited ability to self-renew, proliferate and give rise to more specialised cells
Psoriasis — an inflammatory disease that causes patches of thick, red skin and silvery scales
Signalling cascade — a series of chemical reactions within a cell caused by a certain stimulus
Toll-like receptors (TLRs) — a class of membrane-spanning proteins
DISCIPLINES
Haematology — the branch of medicine involving the study and treatment of blood
Immunology — the branch of medicine and biology that studies the immune system
Medicinal chemistry — the branch of medicine that involves developing molecules for medicines
Microbiology — the branch of science that studies microorganisms
Pharmacology — the branch of medicine involving the study of drugs important to our innate immune system
Inflammation occurs when our cells recognise a pathogen or other foreign molecules. It is a process that ultimately alerts other parts of the immune system to kick into action. “If strong enough, inflammation is what we can feel and, possibly, even see, typically as redness, swelling, heat and pain,” says Professor Hans Haecker. The Haecker Lab at the University of Utah focuses on uncovering the molecular mechanisms behind inflammation and using this knowledge to inform the development of new drugs.
While a certain level of inflammation is normal and useful when encountering a threat to the body, it can lead to problems, too, if it occurs at a large magnitude. “If pathogens enter a person’s bloodstream and promote inflammation in the entire body, the person can become very sick and even die,” says Hans. “Understanding what drives this response and how we can control it is, therefore, critically important.”
Toll-like receptor signalling
Our bodies have various intelligent ways of identifying pathogens. The most well-known are antibodies, but there are also more generalised receptors that act as the first line of defence against pathogens before the highly specific antibodies get involved. “Toll-like receptors (TLRs) are molecules on the surface of cells which recognise certain components of pathogens,” says Hans. “They are transmembrane receptors, which means part of them is on the outside of the cell, to interact with pathogens, and part on the inside, to trigger a response from the cell.”
In humans, there are ten different TLRs that recognise different types of pathogen components. “For example, TLR4 recognises lipopolysaccharides (LPS), which are found on many bacteria,” says Hans. “Another type, TLR9, recognises a special form of DNA found in viruses.” Between them, the TLRs can likely recognise just about any pathogen that exists. Once recognition occurs, the TLR activates a signalling cascade within the cell which ultimately triggers the immune cell response – including the first stage of inflammation.
TLRs and chronic inflammatory diseases
Autoimmune diseases occur when the body’s immune system malfunctions. The inflammation pathway is no exception, and diseases such as lupus and psoriasis happen when the body’s inflammatory response triggers attacks on healthy cells. “For both lupus and psoriasis, there is evidence that the amount of a molecule that forms part of the TLR intracellular signalling cascade, known as ABIN1, is slightly reduced in some patients,” says Hans. “While the precise molecular mechanisms remain unclear, it appears that ABIN1 is essential to tame inflammation, so lower amounts lead to a less controlled inflammatory response.”
Reference
https://doi.org/10.33424/FUTURUM351
Hans’ lab has performed in vivo experiments using mice to test the effects of reduced ABIN1 in more detail. “We’ve found that mice that lack ABIN1 develop lupus- and psoriasis-like diseases,” he says. The team has paired its findings with in vitro experiments that try to uncover the cellular and molecular mechanisms in play. “We can investigate which immune cell types are involved and how we might be able to manipulate them,” says Hans. “This understanding may inform the development of new treatment options.”
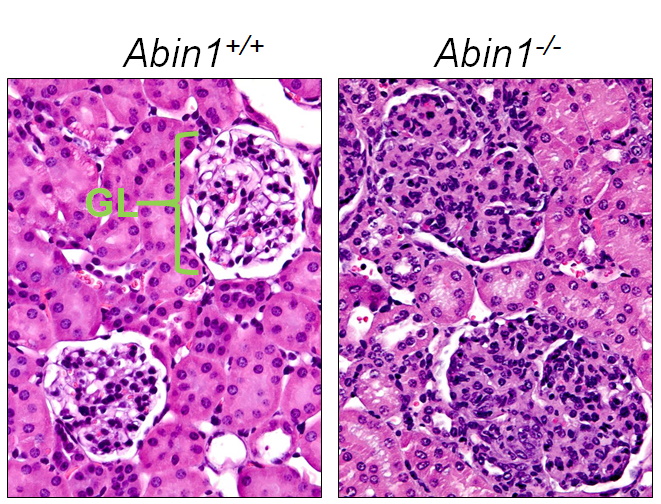
ABIN1-deficient mice develop lupus-like kidney disease. This image is an histology analysis of kidneys from normal (Abin1+/+) and ABIN1-deficient (Abin1-/-) mice. Histology slides are prepared by cutting small parts of mouse kidneys into very thin sections that are mounted on a glass slide and stained with a combination of dyes (hematoxylin and eosin), followed by microscopic analysis. The images show two glomeruli (GL) each, which are made up by specialised blood vessels used to filtrate blood. This fíltrate is excreted (in part) in the urine to get rid of water and unwanted substances. Note the relaxed, airy structure of glomeruli in normal mice and the compressed (defective) structure in Abin1 -/- mice.
While these findings are promising, Hans is quick to add that these diseases are far more complex than an issue with a single molecule. “In lupus, there are dozens of genetic features that suggest a role of specific molecules in the disease process,” he says. “However, it’s important to note that genetic information alone is typically not that informative, but rather hints at which mechanisms might be involved.” For instance, it was such genetic information that suggested the ABIN1 molecule was involved, which led to the experiments where mice were engineered to lack the gene that codes ABIN1, and the effects of this were then monitored.
Drug screening
Drug screening involves the identification of molecules that might have potential as future medicines. “Large numbers of compounds, collectively called a compound ‘library’, are tested to see if any of them have a desired effect,” says Hans. “We use a phenotypic screening approach, which uses living cells and observes their interactions with these compounds.” This approach involves looking for the activation of a certain gene that can be measured, such as those activated by the stimulation of TLRs. “If a compound in your library blocks TLR-driven gene activation, you have a hit,” says Hans.
However, like so much of science, it is not quite that simple. Because the TLR-triggered signalling cascade involves so many different molecules, a screening ‘hit’ does not give you much information on which particular molecule has been affected to break the chain reaction. “To address this, we developed a unique screening platform that breaks the TLR signalling cascade into segments, which we screen consecutively,” says Hans. “This allows us to identify more closely which molecule the compound has affected, simplifying the tedious follow-up work dramatically.”
Interdisciplinary working
Hans’ lab focuses on microbiology and immunology, but the team incorporates expertise from a range of other disciplines. “For example, we are exploring TLR signalling pathways by purifying known components to identify novel molecules,” he says. “This involves an understanding of biochemistry to perform these methods.” The team also needs knowledge on how blood functions and the cells within it – such as immune cells – are produced, which relies on a field called haematology.
While a lot of this know-how comes from within the team, there is a limit. “It would be impossible to have all the expertise required for such large projects combined in one lab,” says Hans. “For instance, for drug development we collaborate with experts in medicinal chemistry and pharmacology, whose contributions are essential.”
Such interdisciplinary working has driven the many successes of the Haecker Lab to date. “We have identified molecules important for cells to detect pathogen DNA, and discovered that this process takes place in little capsules the cell develops to sample molecules from its surroundings,” explains Hans. “We’ve also established a new cell system that allows us to indefinitely keep and multiply ‘progenitor’ immune cells, which can be transferred into mice where they develop into any immune cell type.” These findings and more are substantially adding to the ever-growing knowledge bank of immunology.
 Professor Hans Haecker
Professor Hans Haecker
Fields of research: Microbiology and Immunology
Research projects: Investigating the mechanisms behind innate immunity and inflammation, and consequent drug development
Funders: National Institutes of Health (NIH), University of Utah
ABOUT MICROBIOLOGY AND IMMUNOLOGY
Professor Hans Haecker explains more about his discipline and what enthuses him.
“Microbiology and immunology overlap and complement each other. They are both related to the most important function of the immune system, which is to protect us from pathogenic microbes. While immunology focuses on the host, microbiology focuses on the pathogen. An immunologist may study an immune process (such as TLR function) while a microbiologist may investigate how pathogens trick or overcome our immune systems. Of course, to understand the big picture, it is necessary to consider both together.
“Every day, I get to work with colleagues who are excited by what they do. It is very rewarding in the long term to see the impacts that such collective enthusiasm can have. The field of innate immunology and inflammation has been revolutionised over the last 25 years, not least due to our increased understanding of pathogen receptors such as TLRs. While some of these findings have been translated into medical practice, such as in vaccination strategies, there is much more to be explored.
“Even though we have accumulated an enormous body of knowledge, our understanding of microbiology and immunology is far from complete. Additionally, some newer areas of research, in particular computational biology and approaches driven by artificial intelligence, are becoming more important. Combining new expertise in these areas with expertise in ‘old’ areas has huge potential for the upcoming generation.”
Pathway from school to microbiology and immunology
• Hans recommends studying science subjects such as biology and chemistry at school and post-16. A-level mathematics is often highly desired for university courses, while other science subjects may also be useful.
• At university, genetics and biochemistry remain key. More novel fields, such as computational approaches to analysing large datasets, and integration of AI-driven approaches, are likely to be increasingly important and desirable.
Explore careers in microbiology and immunology
• Both the American Association of Immunologists (AAI) (www.aai.org) and the American Society for Microbiology (ASM) (asm.org) have a range of resources related to outreach, latest scientific findings and career opportunities.
• The University of Utah offers a STEM ambassador programme that provides outreach and insight into science-related careers through camps, lectures, school visits, teacher information and other resources: www.stemap.org/university-of-utah-resources/
• According to CareerExplorer, the average annual salary for an immunologist (full professor) in the US is around $200k.
Meet Hans

I have always loved sports, which typically comes with various injuries! I think this sparked my first career aspiration, which was to become a medical doctor, specifically an orthopaedic surgeon. I first encountered basic research while in medical school. After finishing med school, I felt that having a more robust understanding in science would help me improve my work as a physician. After my PhD and postdoctoral training, prospects in research appeared more promising, so I stayed. However, I’ve remained focused on how to translate research into medicinal practice.
Obtaining funding is always a challenge for academic research, not only for lab equipment and supplies, but also for salaries for your team, including students, postdocs and technicians. NIH provides the lion’s share of research funding in the US, including our own, but we’ve also received funding from specific foundations such as the Lupus Research Alliance and National Psoriasis Foundation.
It’s a privilege to run a lab working on something I find fascinating and believe has real impact. To work towards the end goal to make a real-world difference, through the highs and lows with a dedicated team, is a joy. And the excitement of finding something new never gets old!
I co-founded a company called Nanospot.ai, which focuses on point-of-care diagnostics. These are tests to identify diseases that can be performed at any site, such as with the patient at their home or in a doctor’s office, as opposed to other tests that have to be shipped to reference labs. We developed a test for SARS-CoV2 antibodies, which provides information about the level of immunity a patient has against COVID-19, and combines an ‘old-fashioned’ diagnostic method with AI interpretation. I hope to expand our company further to make diagnostic testing more broadly available and more efficient.
It’s not so common for researchers to set up their own companies. It’s important that the idea behind the endeavour has true commercial potential. You also need the right environment and possibly other co-founders based in industry. If this all comes together, it’s exciting to be part of a start-up and push towards a common goal. A career in research does not have to be restricted to academia.
Hans’ top tips
1. Be curious and open to new information, and choose your own way based on what you find exciting and matches your talents.
2. Be disciplined and don’t be discouraged if things don’t work out right away.
3. Don’t be afraid to ask for help. Most people are happy to support you!
Do you have a question for Hans?
Write it in the comments box below and Hans will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)