Protecting the pancreas
Our immune system is highly organised and ruthlessly efficient, but when it goes wrong it can lead to debilitating disease. Our pancreas is critical for metabolism and digestion. At the Columbia University Irving Medical Center, USA, Dr Stuart Weisberg is studying how immune cells function in both healthy and diseased pancreases, and what this means for treating pancreatic disease.
TALK LIKE AN IMMUNOLOGIST
Antigen — a foreign substance in the body
Genome — the complete set of genes present in an organism
Immune system — the processes and components of the body that provide resistance to threats, such as pathogens
Inflammation — a strong localised immune response to a particular perceived threat
Pancreas — the organ that secretes digestive enzymes into the small intestine and glucose-regulating hormones into the blood
Pathogen — an organism that causes disease, such as bacteria
T cell — a type of white blood cell trained to detect specific pathogens
Tissue-resident memory T cell (TRM) — a T cell that occupies a specific tissue rather than circulating in the blood
Transcriptome — the full range of genes expressed by a cell (specifically, the mRNA transcripts produced)
When we think of our immune system, we usually think of white blood cells patrolling our blood vessels on the lookout for pathogens. In fact, the vast majority of immune cells are not found in the blood but are hunkered down in our organs. “Taking blood is a convenient way to sample immune cells but only gives us a partial picture of the body’s immune system,” says Dr Stuart Weisberg, an immunologist at Columbia University Irving Medical Center. “It’s like if you were studying all of humanity but only examined the people driving around on roads. This would give you a very biased perspective.”
Stuart is building a more detailed picture of how the immune system functions, focusing not on the blood, but instead on one very important organ: the pancreas. “The pancreas is critical for digestion and metabolism,” he says. “It secretes digestive enzymes that break down food in the intestine, as well as releasing hormones that coordinate glucose uptake.” With this in mind, understanding how the pancreas works – and how to restore its function when something goes wrong – is essential.
What has the pancreas ever done for us?
A functioning pancreas is a complex and highly regulated machine. “The pancreas contains cell clusters that produce and secrete powerful digestive enzymes through a network of ducts until they reach the small intestine, where they break down food so the nutrients can be taken up by the body,” explains Stuart. “If the pancreas is damaged or these ducts are blocked, the enzymes can spill out and start digesting the pancreas itself – a painful and sometimes fatal disease known as pancreatitis.”
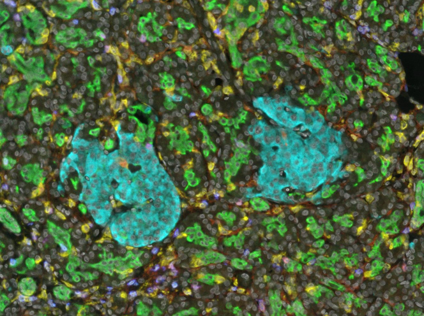
As well as enabling digestion, the pancreas secretes hormones into the blood to control how the body uses sugars. “Small islands of cells (called ‘islets’) within the pancreas sense blood glucose levels and secrete hormones (insulin and glucagon) into the blood to coordinate glucose uptake by all cells in the body,” says Stuart. If these cells are disturbed, we lose our ability to regulate our glucose levels. For instance, Type 1 diabetes occurs if the immune system attacks these cells, while Type 2 diabetes develops if these cells suffer chronic stress due to aging or obesity. “Individuals with diabetes must monitor and carefully control their blood glucose levels at all times, as the pancreas doesn’t do it for them,” explains Stuart.
What role do T cells play?
Stuart and his team are interested in how the pancreas regulates its own specific section of the immune system. “T cells are highly specialised cells of our immune system that protect the body from infection and cancer,” he explains. “Each T cell has a unique receptor (a ‘T cell receptor’) that can recognise a very specific molecule (an antigen) as foreign, such as those carried by invading pathogens.” Once the T cell receptor recognises its target, the cell replicates vigorously to detect and destroy all cells in the body that contain this antigen. Once the threat is removed, most of these T cells die, but a small number remain as our immune ‘memory’, ready to quickly detect and replicate if the antigen shows up again. In addition, T cells cannot work alone. Their recognition of antigens requires the antigens to be processed and presented by other cells in the tissue.
Most memory T cells reside in our organs as tissue-resident memory T cells (TRMs). “TRMs are adapted to survive, function and be retained in a specific tissue site,” says Stuart. “They help our organs remember past infections and provide a localised defence against pathogens and cancer.” Every tissue – from our skin to our lungs to our brain – needs TRMs, but every tissue is also different, each with unique requirements for immunity that are dictated by the anatomy and function of the cells in the tissue. Stuart’s research is focused on how TRMs function in the pancreas.
How does Stuart study pancreatic TRMs?
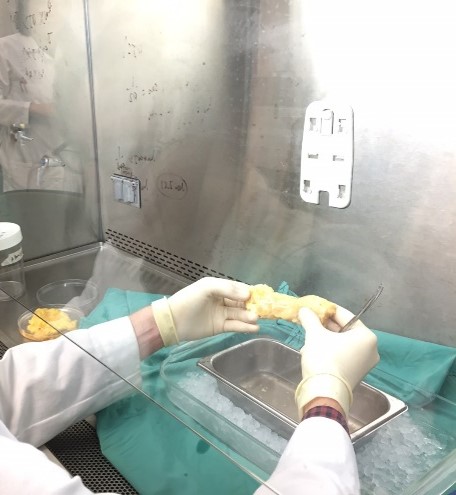
The study of human pancreas cells relies on the generosity of organ donors. When organs from recently deceased donors are not needed or cannot be used for transplanting, they can, with consent, be used for research instead. However, unearthing the systems behind pancreas functioning is no easy task. “Obtaining live cells from the pancreas is difficult because, once the clusters containing digestive enzymes are disrupted, the organ begins digesting itself,” explains Stuart. “The separation of the pancreas into its component parts has to be performed rapidly, gently and with careful controls.” Stuart’s team has refined methods for doing this to place as little stress on cells as possible, so Stuart can observe pancreas cell function in a way that minimises the disruption to the cells.
Reference
https://doi.org/10.33424/FUTURUM379
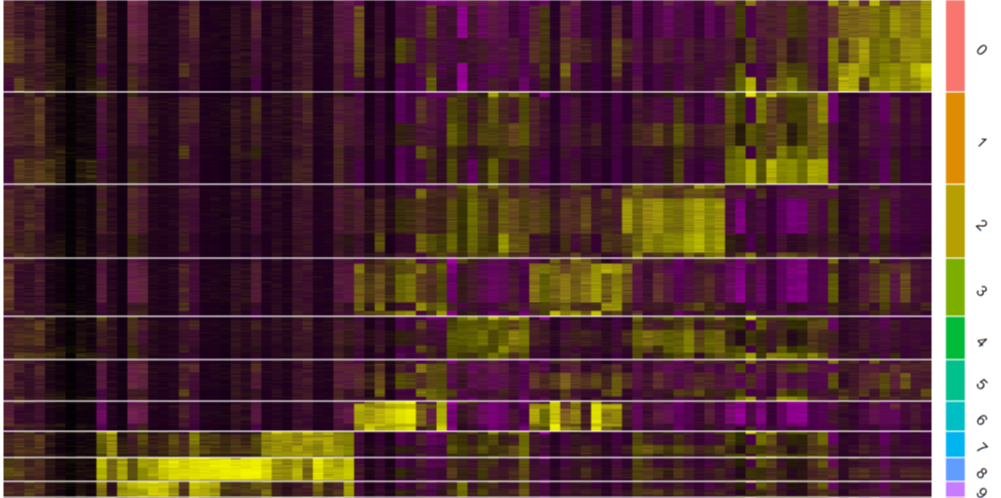
Once Stuart has isolated living pancreatic cells, such as TRMs, he studies them using a technique called transcriptome profiling. Our genome describes the entirety of our DNA and all the genes within it and is the same for every cell in our body. Our transcriptome, however, only describes the genes that are actively being used by a specific cell, which depends on its environment and the stimuli it is receiving. Transcriptome profiling involves cataloguing all the activated genes in a cell, including the degree to which they are being activated in any particular scenario.
What has Stuart discovered?
Stuart’s work in transcriptome profiling has revealed fascinating insights about pancreatic TRMs. “The most interesting features of these cells are the ones that help them do their job without causing excessive damage to surrounding pancreatic tissue,” he says. “Pancreatic TRMs can release powerful toxic molecules capable of directly killing cells and can also make molecules that summon other immune cells and induce inflammation.” While these features are great when they are needed – when the pancreas is under attack from a pathogen, for instance – they must be kept carefully in check to avoid damaging the pancreas’ own cells.
Pancreatic TRMs have a surface receptor specific to a molecule released by neighbouring cells that, when activated, restrains TRM responses. “Just like well-trained police dogs, these cells have great potential to kill invaders and call in back up, but when not needed, they remain calm and functionally restrained.” Stuart’s team has found that, in patients with chronic pancreatitis, it is this relationship that is malfunctioning, leading to TRMs being abnormally activated and provoking an unneeded immune response that constantly inflames and damages the pancreas.
From research to healthcare
“As physicians, we often dismiss the biology of healthy organs as they are not causing problems for our patients,” says Stuart. “But when we think about it, the maintenance of healthy organs over decades in a danger-filled environment is a remarkable process worthy of intense research.” The study of medicine usually focuses on when things go wrong, but Stuart is adamant that understanding healthy organ function is just as, if not more, important. For every discipline, from medicine to automobile mechanics, understanding how something works when it is going right is essential for getting things back on track when something goes wrong.
This is why Stuart’s team is examining TRM function in both healthy and unhealthy pancreatic tissue. “It is important that we understand how the healthy pancreas strikes a balance between robust immune defence and avoiding damaging inflammation, because many pancreatic diseases are caused by disruption of that balance,” says Stuart. “Excessive TRM activation causes inflammation which can lead to pancreatitis and diabetes, but if TRMs are not activated sufficiently, the pancreas can suffer from destructive infections or develop cancer.” Stuart’s research has found that a healthy pancreas has abundant resident immune cells, which are maintained in a very specific state and dispersed in certain ways throughout the pancreas. “By defining the specific cells and signalling pathways that maintain this state, we can learn how to better restore health to patients with pancreatic disease,” he says.
 Dr Stuart Weisberg
Dr Stuart Weisberg
Assistant Professor of Pathology and Cell Biology, Columbia University Irving Medical Center, USA
Field of research: Immunology
Research project: Examining the function of tissue-resident memory T cells in the pancreas
Funders: National Institutes of Health (NIH): National Institute of Diabetes and Digestive and Kidney Diseases, Gerstner Family Foundation
About immunology
Immunology is the study of our body’s immune system, including how it responds to threats and what happens when it malfunctions. Immunology is essential for developing effective healthcare for existing and novel diseases and is responsible for directly saving countless lives.
“The human immune system is shaped by the pathogens it encounters, and some of the most important sites for immune defence are the interfaces between the body and environment: our airways, lungs and gastrointestinal tract,” says Stuart. “If our environment changes, our immune system has to adapt.”
How is globalisation impacting our immune systems?
Societal developments can cause significant changes to the threats that our immune system encounters. For instance, urbanisation exposes us to more air pollution, climate change is altering the number, variety and distributions of pathogens, and habitat destruction is causing humans to come into closer contact with animal diseases.
“Immune responses require a lot of energy to function, so rely on good nutrition,” says Stuart. “All forms of malnutrition – over nutrition and under nutrition – impair immune responses.” Globalisation has increased nutritional disparities, with some people not having enough to eat and others over-consuming unhealthy processed foods. Our interconnected modern world also means that pathogens can spread rapidly as people travel great distances between countries, potentially carrying diseases with them.
What are the joys of immunology?
While recognising the importance of his work, Stuart also finds it extremely enjoyable and fulfilling. “One of the best aspects of immunology is that, as immune cells interact with all other cell types in the body, it invites exciting collaborations with many different scientists,” he says. Stuart is a physician scientist, so alongside his immunology research, he also works as a medical doctor in a hospital. “As a physician scientist, I’m conscious that my immunology research needs to be directly relevant to potential treatments for my patients in hospital,” he says. “Organ transplantation, treating cancer and fighting infectious diseases all rely on immunology.”
Pathway from school to immunology
• Study biology, chemistry and mathematics at school and college, as these will teach you the fundamental knowledge behind immunology.
• Some universities offer undergraduate or postgraduate degrees in immunology. Studying biology or molecular biology could also lead to a career in the field.
• “You need to understand molecular biology and molecular genetics to understand immunology,” says Stuart, so take as many classes as possible in these areas.
• Immunologists commonly analyse large datasets, so take courses that will teach you the skills required to do this. “Learn how to code in various computer languages and learn the statistical methods to create models from these large datasets,” Stuart advises.
Explore careers in immunology
• Some immunologists are medical doctors who work with patients to diagnose and treat illnesses related to the immune system. Others are academic scientists who conduct research to improve our understanding of the immune system and what happens when it malfunctions.
• Columbia University Irving Medical Center has a rich array of community programmes and initiatives, many aimed at inspiring young people towards medical careers by providing opportunities to participate in research: www.gca.cuimc.columbia.edu/community-service-programs/community-programs-initiatives
• This blog describes the steps to become an immunologist and explains what a career in the field involves: www.inspiraadvantage.com/blog/how-to-become-an-immunologist
Meet Stuart
I didn’t always want to be a doctor. I was originally interested in journalism rather than medicine. However, I soon discovered I enjoyed the investigative aspect of journalism more than the writing, which morphed into a desire to pursue the investigative side of medicine.
As a medical student, I worked in a research laboratory full of friendly, open-minded and rigorous scientists. I enjoyed the experience so much that I transitioned from aiming for a career as a medical doctor to instead becoming a physician scientist. That lab was also where my interest in tissue immunity began, and our interactions with the lab next door primed my interest in pancreas tissue immunity.
I have had a series of outstanding mentors who have inspired me. They have opened many doors to help me develop my career and collaborate with a diverse community of dedicated scientists, without which my studies in pancreas tissue immunity would not be possible.
As a physician scientist, I enjoy it when my research background helps me provide novel insights that inform my clinical work. I can help patients understand their medical problems at a more fundamental level and therefore feel more comfortable with their treatment plan.
The most challenging aspect of my dual roles as a physician scientist is switching from one to the other. Working with patients in the hospital is nothing like working in a research lab, and sometimes it can feel incompatible. At these times, patient care always comes first, even at the expense of research.
Outside of work, I enjoy spending time with my wife and children and our pet hamster, Bernie. We love doing anything outside, such as camping, swimming, hiking and biking. We enjoy live music, visiting relatives and travelling.
Stuart’s top tips
1. Don’t try to follow in anyone’s footsteps. Make your own footsteps, instead.
2. We should always aim to improve on what has come before us, using lessons and insights from others to figure out new and unexpected things.
3. Immunology and medicine are complex fields that can seem overwhelming, so pace yourself and don’t think you have to understand everything immediately.
Do you have a question for Stuart?
Write it in the comments box below and Stuart will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)


Good evening Dr. Very interesting topic you explained, it intrigues my mind. Myself as a traditional medicine scientist in South Africa looking at active natural compounds, phytochemicals able to flush the pancreas and other organs on a molecule level for their better functioning, after many years of it not been serviced but only been overloaded with process foods until its completely out of balance. There are natural products intervention that can purge the pancreas to redefine or get its balance again and start doing its work again, but in very complex alternative herbal formulations. So maintenance of each organ very important, but most individuals wait for the GI-Tract to go off balance completely and start compromising other organs putting their lives at risk.
Thank you for your comment Dee. We wish you well in your work.
Futurum Webmaster