Shining a light on the role of trace metals in neurodegenerative diseases
A team of researchers with expertise in physics, engineering and biology are finding out whether trace metals in the brain are linked to neurodegenerative diseases. Using innovative synchrotron techniques at Diamond Light Source, the UK’s national synchrotron facility, their work is paving the way for improved diagnosis and treatment
GLOSSARY
METALLOMICS – the study of the role of metal elements in living systems
HOMEOSTASIS – the regulation of conditions in the body such as temperature, water content, carbon dioxide, glucose and levels of chemical elements (www.bbc.co.uk/bitesize/guides/z4khvcw/revision/1)
DEMENTIA – a group of related symptoms (such as memory loss and difficulties with problem-solving or language) associated with an ongoing decline in brain functioning
ALZHEIMER’S DISEASE – the most common form of dementia, leading to symptoms such as memory loss
PARKINSON’S DISEASE – a neurodegenerative disease that includes loss of nerve cells in part of the brain called the substantia nigra
AMYLOID PLAQUES – insoluble protein-rich deposits, including assembled fibres of amyloid protein
TRANSITION METALS – metals in the central block of the periodic table, including iron
TRACE METALS – metals that are present in small but measurable concentrations in animal and plant cells and tissues
MAGNETIC RESONANCE IMAGING (MRI) – a medical imaging technique that uses the magnetic properties of biological tissue to look in detail at anatomy and can identify and locate some ions and molecules
BEAMLINE – an experimental station at a facility, where a beam emitted from a particle accelerator (such as photons at a synchrotron facility) is used for a particular type of measurement (e.g diffraction, tomography, fluorescence)
BIO-AVAILABLE METAL – a measure of the degree to which a metal ion or metal compound can be used in biochemical reactions
According to the World Health Organization (WHO), around 25-30% of people aged 85 or older have dementia. Indeed, diseases such as Alzheimer’s and Parkinson’s are an increasing problem in ageing populations worldwide, but it is not only the older populations who are suffering from dementia: around 1 in every 20 cases of Alzheimer’s disease affects people aged 40 to 65. Finding out the causes of dementia, and how to cure or slow the development of neurodegenerative diseases such as Alzheimer’s and Parkinson’s, is therefore crucial.
Surprising as it may seem, the human brain (and body) is full of trace metals. These metals are essential if our brains are to work properly. For example, the human brain contains about 6 milligrams of copper, enough to produce a small circuit board! Higher-than-usual levels of iron were first reported in the brains of people with Alzheimer’s disease in 1953. Since then, researchers have discovered altered concentrations of trace metals such as copper, zinc and iron in certain regions of the brains of deceased Alzheimer’s patients – and many of these metals have been found concentrated in amyloid plaques. It is known that when there is an imbalance of metals in the body, things start to go wrong. But it is not yet fully understood how changing metal levels affect the onset or progression of dementia.
HOW DO WE FIND OUT?
Professor Joanna Collingwood is the Head of the Trace Metals in Medicine Laboratory at the University of Warwick. She is collaborating with a team of researchers with physics, biology and engineering expertise to investigate the role trace metals play in the development of Alzheimer’s and Parkinson’s disease. They are using a state-of-the-art synchrotron technique, called X-ray spectromicroscopy, to image and analyse the brain in innovative ways.
“We need to think about the brain as a dynamic system, about the balance (homeostasis) being disrupted, to determine if too much of one metal, or not enough of another, is preventing cells from functioning properly,” says Jo. “When we study neurodegenerative diseases that happen in humans, we normally look at brain tissue that has been donated to a brain bank after someone’s death. This means we’re looking at evidence from a single point in time. To try and understand the timeframe over which chemical changes in the brain have happened (quickly, or slowly – maybe over decades), we have to use evidence from medical imaging studies (where available).”
WHY IS IT IMPORTANT TO STUDY CHANGES IN METAL HOMEOSTASIS?
Studies of post-mortem brains have revealed that iron concentrations change as we get older. However, changes in metal chemistry appear to be more marked in diseased brains than in healthy brains. “People with Alzheimer’s disease may exhibit metal-rich deposits of proteins, called plaques, in their brain tissue,” Jo explains. “Meanwhile, with Parkinson’s, concentrations of iron in some vulnerable neurones are higher compared to healthy individuals of the same age.”
Jo continues: “Because the changes in metal homeostasis depend on the type of disorder, we think it is important to describe these changes as well as possible. Firstly, because it might be possible to detect these changes with medical imaging techniques (such as magnetic resonance imaging (MRI), which can be sensitive to iron), to help obtain an earlier and/ or more accurate diagnosis. Secondly, because a better description of how brain chemistry is changing in these disorders might create opportunities for better treatment.”
Reference
https://doi.org/10.33424/FUTURUM259
GLOSSARY
METALLOMICS – the study of the role of metal elements in living systems
HOMEOSTASIS – the regulation of conditions in the body such as temperature, water content, carbon dioxide, glucose and levels of chemical elements (www.bbc.co.uk/bitesize/guides/z4khvcw/revision/1)
DEMENTIA – a group of related symptoms (such as memory loss and difficulties with problem-solving or language) associated with an ongoing decline in brain functioning
ALZHEIMER’S DISEASE – the most common form of dementia, leading to symptoms such as memory loss
PARKINSON’S DISEASE – a neurodegenerative disease that includes loss of nerve cells in part of the brain called the substantia nigra
AMYLOID PLAQUES – insoluble protein-rich deposits, including assembled fibres of amyloid protein
TRANSITION METALS – metals in the central block of the periodic table, including iron
TRACE METALS – metals that are present in small but measurable concentrations in animal and plant cells and tissues
MAGNETIC RESONANCE IMAGING (MRI) – a medical imaging technique that uses the magnetic properties of biological tissue to look in detail at anatomy and can identify and locate some ions and molecules
BEAMLINE – an experimental station at a facility, where a beam emitted from a particle accelerator (such as photons at a synchrotron facility) is used for a particular type of measurement (e.g diffraction, tomography, fluorescence)
BIO-AVAILABLE METAL – a measure of the degree to which a metal ion or metal compound can be used in biochemical reactions
According to the World Health Organization (WHO), around 25-30% of people aged 85 or older have dementia. Indeed, diseases such as Alzheimer’s and Parkinson’s are an increasing problem in ageing populations worldwide, but it is not only the older populations who are suffering from dementia: around 1 in every 20 cases of Alzheimer’s disease affects people aged 40 to 65. Finding out the causes of dementia, and how to cure or slow the development of neurodegenerative diseases such as Alzheimer’s and Parkinson’s, is therefore crucial.
Surprising as it may seem, the human brain (and body) is full of trace metals. These metals are essential if our brains are to work properly. For example, the human brain contains about 6 milligrams of copper, enough to produce a small circuit board! Higher-than-usual levels of iron were first reported in the brains of people with Alzheimer’s disease in 1953. Since then, researchers have discovered altered concentrations of trace metals such as copper, zinc and iron in certain regions of the brains of deceased Alzheimer’s patients – and many of these metals have been found concentrated in amyloid plaques. It is known that when there is an imbalance of metals in the body, things start to go wrong. But it is not yet fully understood how changing metal levels affect the onset or progression of dementia.
HOW DO WE FIND OUT?
Professor Joanna Collingwood is the Head of the Trace Metals in Medicine Laboratory at the University of Warwick. She is collaborating with a team of researchers with physics, biology and engineering expertise to investigate the role trace metals play in the development of Alzheimer’s and Parkinson’s disease. They are using a state-of-the-art synchrotron technique, called X-ray spectromicroscopy, to image and analyse the brain in innovative ways.
“We need to think about the brain as a dynamic system, about the balance (homeostasis) being disrupted, to determine if too much of one metal, or not enough of another, is preventing cells from functioning properly,” says Jo. “When we study neurodegenerative diseases that happen in humans, we normally look at brain tissue that has been donated to a brain bank after someone’s death. This means we’re looking at evidence from a single point in time. To try and understand the timeframe over which chemical changes in the brain have happened (quickly, or slowly – maybe over decades), we have to use evidence from medical imaging studies (where available).”
WHY IS IT IMPORTANT TO STUDY CHANGES IN METAL HOMEOSTASIS?
Studies of post-mortem brains have revealed that iron concentrations change as we get older. However, changes in metal chemistry appear to be more marked in diseased brains than in healthy brains. “People with Alzheimer’s disease may exhibit metal-rich deposits of proteins, called plaques, in their brain tissue,” Jo explains. “Meanwhile, with Parkinson’s, concentrations of iron in some vulnerable neurones are higher compared to healthy individuals of the same age.”
Jo continues: “Because the changes in metal homeostasis depend on the type of disorder, we think it is important to describe these changes as well as possible. Firstly, because it might be possible to detect these changes with medical imaging techniques (such as magnetic resonance imaging (MRI), which can be sensitive to iron), to help obtain an earlier and/ or more accurate diagnosis. Secondly, because a better description of how brain chemistry is changing in these disorders might create opportunities for better treatment.”
WHAT HAPPENS WHEN X-RAYS INTERACT WITH METALS IN THE BRAIN?
The elements that make up the biological tissue in the brain have different arrangements of electrons around their central nuclei. This gives each element a unique set of energy levels – a signature that shows up when X-rayed. When an X-ray beam interacts with a sample of brain tissue, only those elements with an excitation energy at or below the energy of the X rays will exhibit fluorescence. “Scientists have already made lists of energies that are absorbed by different elements, so we can identify the different chemical elements with high accuracy,” says Jo.
Biomedical imaging has progressed in leaps and bounds in recent years thanks to technological advances, but imaging changes in metal homeostasis is challenging. Conventional imaging techniques are not sensitive enough to detect low concentrations of certain metals, to differentiate between the various trace metals and their chemical properties. Moreover, these techniques can damage or change the metal chemistry within brain samples. Jo and the team are taking advantage of X-ray spectromicroscopy and other imaging techniques to advance our understanding of the brain in new ways.
WHAT IS SPECTROMICROSCOPY?
“Synchrotron X-ray spectromicroscopy is fantastic because it can be used without damaging the sample. It is very accurate for identifying the different elements, and can be used on samples that are as close as possible to their ‘native state’ to preserve their properties,” says Jo. “Spectromicroscopy is not commonly used to study brain tissue, so there is still a lot to discover and develop in the way that we can apply this approach.” So, what is spectromicroscopy?
You can think of spectromicroscopy as a series of maps overlaid one on top of the other, like different maps of a city. One map might show where the shops are, another where the train stations are, a third where the parks are. Eachof these maps is useful on their own, but toreally understand how a city works you needto know, for example, whether train stationsare close to shops or parks close to restaurants. In a similar way, spectromicroscopy not only allows scientists to see the distributionsof metals such as iron, copper and zincindividually, but also to investigate thecorrelation between the three distributions.
Spectromicroscopy also contains detailedinformation about how the X-rays havebeen absorbed by the elements, allowing the scientists to extract detailed information about individual features. This is what makes the technique more powerful than other element-mapping methods. In the city map example, this might be the equivalent of not only seeing the relative location of facilities such as stations and restaurants, but also being able to see their opening hours and customer reviews. “Spectromicroscopy not only allows scientists to see the relationship between the element distributions, it also allows investigation of individual elemental properties, such as chemical state, crystal structure and magnetic properties,” Jo explains.
WHAT DOES JO’S RESEARCH MEAN FOR DIAGNOSTICS AND THE TREATMENT OF NEURODEGENERATIVE DISORDERS?
“The work we do is contributing small pieces of information into a huge and complex jigsaw of knowledge,” says Jo. Indeed, a better understanding of trace metals’ role in the development of neurodegenerative disorders such as Alzheimer’s and Parkinson’s will certainly help clinicians make earlier and more accurate diagnoses. For example, if higher concentrations of iron in the brain are found to be linked with these diseases, then MRI, which can be sensitive to iron, might be used to detect changes in iron levels.
The information that the team is gathering will also pave the way for better treatments. “There are drugs that can bind metals and remove them from tissue if levels are too high, and this might protect cells,” says Jo. “But, if the problem is that a metal element is accumulating in one particular type of cell, causing shortages elsewhere, then lowering levels in the tissue with a drug might not solve the problem.”
What is exciting is that the team is able to use synchrotron techniques that were originally developed for other applications. “By using unusual techniques, we are able to approach really difficult questions from different perspectives,” Jo concludes. “This can encourage new ways of thinking about problems, and hopefully help us move understanding forward in areas where progress is urgently needed.”
Head of Trace Metals in Medicine Laboratory, School of Engineering, University of Warwick, UK
FIELD OF RESEARCH: Metallomics, Biomedical Engineering
RESEARCH PROJECT: Using spectromicroscopy and other techniques to understand changes in brain chemistry linked to neurodegenerative disease
FUNDERS: Economic and Social Research Council (ESRC), UK: Engineering and Physical Sciences Research Council (EPSRC), Diamond Light Source, LGC Ltd., Alzheimer’s Society
 PROFESSOR JOANNA COLLINGWOOD
PROFESSOR JOANNA COLLINGWOOD
Head of Trace Metals in Medicine Laboratory, School of Engineering, University of Warwick, UK
FIELD OF RESEARCH: Metallomics, Biomedical Engineering
RESEARCH PROJECT: Using spectromicroscopy and other techniques to understand changes in brain chemistry linked to neurodegenerative disease
FUNDERS: Economic and Social Research Council (ESRC), UK: Engineering and Physical Sciences Research Council (EPSRC), Diamond Light Source, LGC Ltd., Alzheimer’s Society
MEET JO

I did my first synchrotron experiments on metallomics at a facility in Chicago. The site there is out in the windswept countryside, and gets very cold in winter. Arriving there in the dark of night, I spotted coyotes as well as deer crossing the site!
When I was a teenager, I wanted to be an archaeologist and I studied history and art as well as maths and physics at A-level. I eventually decided that, whilst I could always enjoy history or art as a hobby, I wanted to study either physics or engineering at university. The advice I got was that I could always move from physics to engineering, but that it would be harder to move the other way. I took physics, and now find myself working in an engineering department, so perhaps this was good advice!
Studying physics can give you tremendous opportunities, allowing you to delve deep into the origins and matter of the universe around us. At the same time, it creates a strong foundation to study other subjects – personally, I really enjoy expanding my knowledge of biology and chemistry from the perspective of having studied physics to postgraduate level.
JO’S TOP TIPS
01 Do something that interests you and that you are curious about.
02 Try to find an environment where you are supported to reach your potential.
03 There is no pre-defined formula for success – I wish that when I was younger I had known how much scope for creativity and variety there would be in my career!
MEET THE TEAM

PROFESSOR NEIL TELLING
RESEARCH DIRECTOR AND PROFESSOR OF BIOMEDICAL NANOPHYSICS, SCHOOL OF PHARMACY AND BIOENGINEERING, KEELE UNIVERSITY, UK
I first started working with synchrotrons to study the artificial materials used in technologies such as computer storage. Over time, I realised that many of the techniques we were using could also be applied to biological materials and, for the past ten years, I have been working with Jo to develop these techniques for the study of metal deposits in brain tissue.
Performing a synchrotron experiment is intense, but it can also be an exciting and rewarding experience. A typical experiment may only last a few days, but over that period we work on a 24-hour rota to make best use of time. I have had many ‘Eureka’ moments, often in the small hours of the morning, when we have discovered some fundamental new science. In most cases, these breakthroughs would be impossible without access to the state-of-the-art labs and equipment available at a synchrotron.
After initially studying physics, I was based in an Earth Sciences department at a university for some time, studying how bacteria use metals to form magnetic materials on a very small scale. Some of the synchrotron techniques we are now using to study Alzheimer’s disease were originally developed from this earlier work trying to understand bacteria.
I had a very good physics teacher at school. They helped me understand physics at a fundamental level and grasp the basic concepts of the subject. I see physics as being very powerful because it overlaps with many other areas of science, from astronomy to Earth sciences to medicine. It also trains you to be both systematic and creative in the way you approach problem solving – which is important in your life outside science, too!
A background in physics gives you incredible flexibility to move into different fields. For example, as a child, I was interested in becoming a doctor, then went down a different route, but due to my training in physics I have ultimately ended up being able to work in medical sciences!

DR JAMES EVERETT
RESEARCH ASSOCIATE, SCHOOL OF PHARMACY AND BIOENGINEERING, UNIVERSITY OF KEELE, UK
In my role as a research associate, I am mainly responsible for co-ordinating and conducting novel experiments. Following the completion of the experiments, I analyse and interpret our results, before reporting our findings in scientific journals, in news articles and at conferences.
We make exciting new discoveries about the brain quite consistently, thanks to the unique chemical and spatial sensitivity which synchrotron techniques offer. For example, we recently discovered extremely small particles of metal iron and copper (approximately 1/10000th the size of a pinhead) in human brain tissue for the first time. Until now, only metal oxide forms had been observed.
The discoveries we make about metals in the brain also suggest new ways to diagnose disease. By using MRI scans to spot iron accumulation in the brain, we may be able to identify people with Alzheimer’s at an earlier stage in the disease, in turn allowing us to start treating them sooner.
I chose to study biology because I had a particular interest in how the bodies of humans and other animals work. Whilst at university, I spent time volunteering in research labs, where I had the opportunity to witness some ground breaking experiments in biology, and this work has real potential to improve people’s lives. This inspired me to go on to postgraduate biology research.
Biology is an incredibly diverse area, with plenty of room to find a topic where you have a deep interest. I would recommend a career in biology for anyone with an interest in the natural world!

DR JAKE BROOKS
RESEARCH FELLOW, SCHOOL OF ENGINEERING, UNIVERSITY OF WARWICK, UK
One of my roles is to prepare the brain tissue samples before they undergo synchrotron analysis. Since I finished my PhD, I have been a research fellow in the Trace Metals in Medicine group at Warwick, where I spend much of my time doing hands-on work in the Trace Metals Lab. However, I am also a core member of the synchrotron data collection team, regularly participating in experiments at Diamond Light Source.
I enjoy learning from more experienced members of the team as we collect data from the synchrotron, sharing in the highs and lows of the experiments. Spending time at a synchrotron facility is also a chance to escape the normal lab environment and experience a change of scenery. The Advanced Light Source near San Francisco in California is particularly scenic, situated at the top of a steep hill overlooking the bay with the Golden Gate Bridge in the distance!
As someone with a background in materials engineering, I am enjoying the process of learning more about neurology. My training equipped me with some fundamental knowledge of microscopy techniques, which I am now applying in a biological context. In my current research, I am particularly interested in the disruption to brain metal chemistry which takes place during Parkinson’s disease. With age-related neurodegenerative diseases set to become an increasing problem in the ageing population, I feel there is scope to make a significant impact working in this area.
I have always been fascinated by medicine, even though I didn’t study biology or chemistry at A-level. So, after going to university to study materials engineering, I specialised in biomedical engineering for my PhD.
The skills in microscopy and image analysis, which I was provided with during my materials engineering degree, have proved highly valuable to my current research. You never know where your chosen subject might take you!
I think it is vital that we promote diversity in STEM careers and bring as many different perspectives as possible to tackling some of the world’s biggest scientific challenges. I believe it is important that there are no barriers to higher education and that there should always be a pathway for those who wish to follow an academic route.

DR TINA GERAKI
BEAMLINE SCIENTIST, DIAMOND LIGHT SOURCE, UK
My position at Diamond Light Source means I can offer more specialised X-ray expertise as part of the team. This complements Jo’s broader focus on the big biological questions we can investigate using our instruments.
It is my responsibility to prepare the instruments, so my role bridges the worlds of engineering, physics and biology.
As a beamline scientist I get to work with visiting scientists from a variety of research areas – so I can learn something new every day! Facilitating the thrill of a new discovery, however small, is a worthy reward for the sometimes long and stressful hours spent chasing photons.
In the past few years, it has been refreshing to see many young scientists and engineers join Diamond Light Source. Our staff are diverse, making Diamond a uniquely exciting place to work.
I originally studied physics at university, then specialised in medical physics for postgraduate studies. During the first year of my PhD, I visited the ESRF in France, and at the end of my three-day visit I knew this was the route I wanted to pursue.
I am still fascinated by invisible particles, but, at the same time, I have developed an interest in biological questions. Finding how to make the two disciplines meet in the most productive way possible is what drives me.

NATALIE ARIGUNDIYA
PHD STUDENT, ANALYTICAL SCIENCE CENTRE FOR DOCTORAL TRAINING (AS-CDT), UNIVERSITY OF WARWICK AND DIAMOND LIGHT SOURCE, UK
My role in the team is to lead a project to understand a rare form of Parkinson’s disease, called progressive supranuclear palsy. I will use the microfocus beamline to map metal element distributions in human brain tissue samples at Diamond Light Source, and I will be supervised jointly by Tina at Diamond and Jo at Warwick.
This research pushes me out of my comfort zone by mixing physics, biology and chemistry. So far, I’ve been using very standard equipment to address my difficulties and conduct my research. Working at The Diamond Light Source alters all of that, allowing me to work with a more complex set of equipment that provides far greater resolution than typical laboratory microscopes.
Throughout my bachelor’s and master’s degrees, I became very comfortable in the laboratory setting, which allowed me to hone my problem-solving, data-handling, and communication abilities.
I thoroughly loved studying biomedical science and would strongly recommend it. Biomedical science helped me discover where my interests were. My studies in cellular biology, neurology, molecular genetics, immunology, anatomy and virology enabled me to explore many routes before deciding on what I wanted to specialise in.
There are plenty of careers that you may have not heard off. For those reasons going to careers fairs and networking is very beneficial. More often than not, networking often gets you in the door.
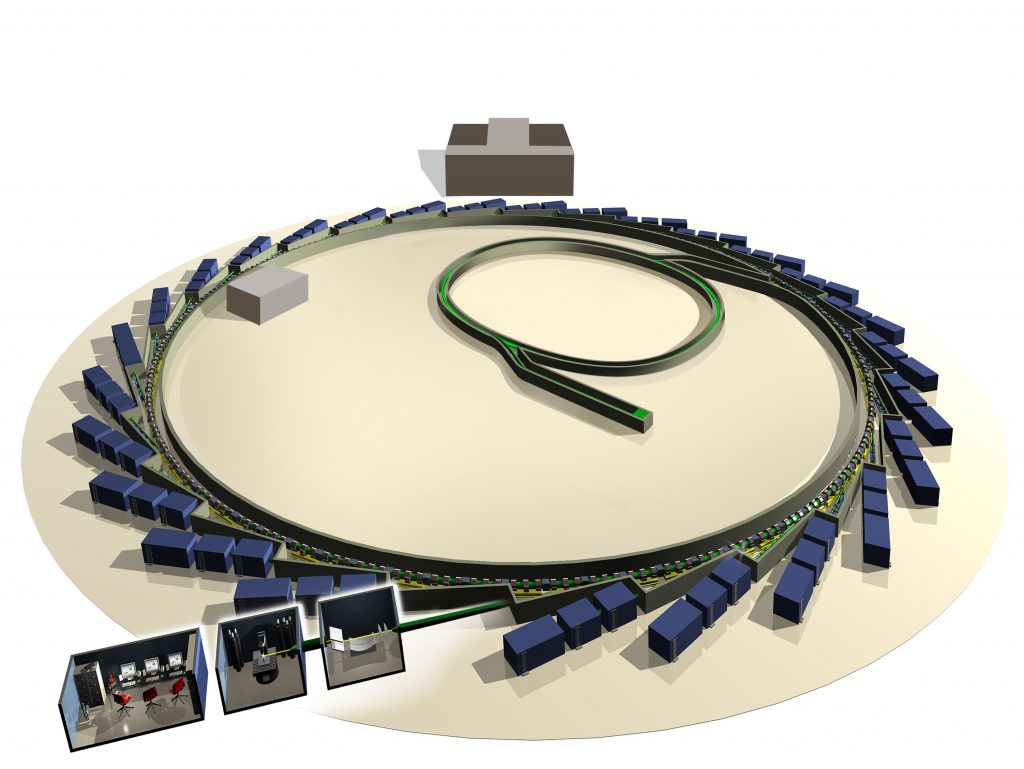
ABOUT SYNCHROTRONS AND DIAMOND LIGHT SOURCE
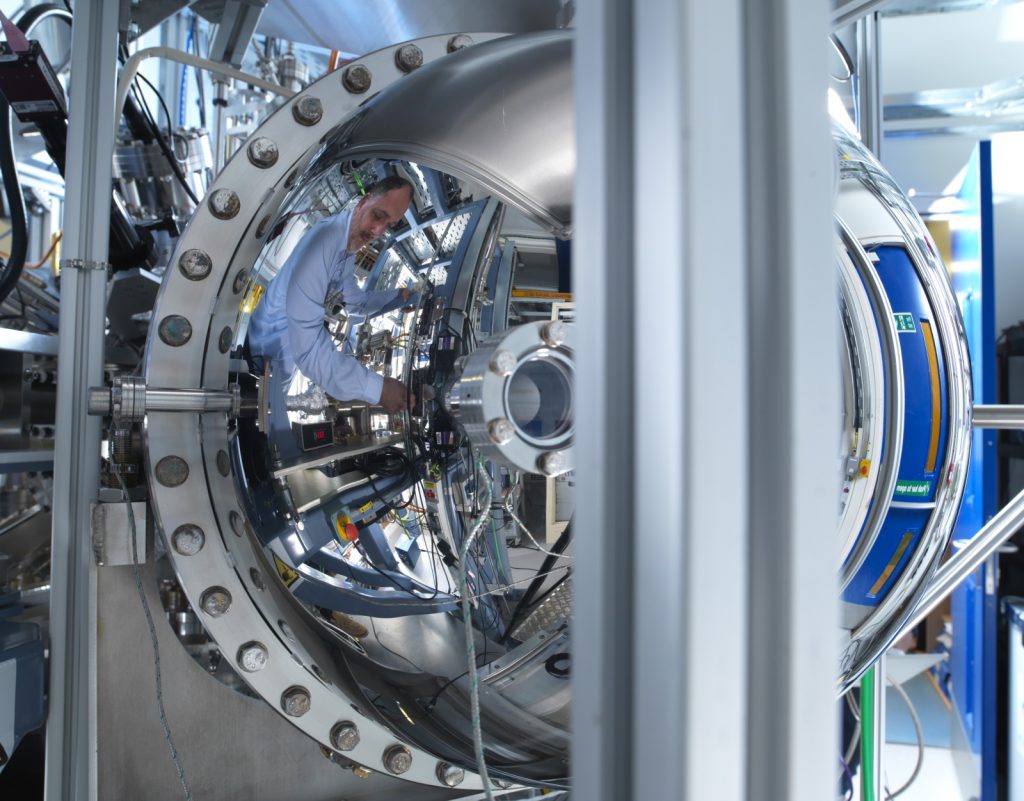
Diamond Light Source is the UK’s national synchrotron facility, located in Oxfordshire. A synchrotron is a type of circular particle accelerator that works like a giant microscope, harnessing the power of electrons to produce light that is 10 billion times brighter than the Sun! These bright beams of light are delivered to instrument stations known as beamlines, which scientists can use to study anything from fossils to jet engines, viruses to ancient paintings – and to brain tissue. Over 14,000 researchers from academia and industry currently use Diamond Light Source.
As well as operating Diamond Light Source, the UK is also a member of the European Synchrotron Radiation Facility (ESRF) in France. There are more than 50 synchrotrons in the world that are either in operation or under construction.
Jo has worked with synchrotrons in Switzerland, France, and in Chicago and California in the US. She tells us more.
HOW LONG HAVE YOU BEEN WORKING WITH SYNCHROTRONS?
Since I was a PhD student, when I did my first synchrotron experiments at the ESRF in Grenoble (measuring the magnetic properties of metal alloys).
ARE SYNCHROTRONS DANGEROUS TO WORK WITH?
No. In principle they could be if you were exposed to the X-ray beam, but in practice there are multi-stage safety systems in place so that you should never be shut into the enclosed experiment chamber (the ‘hutch’) when the beam is on.
WHAT DO YOU LOVE ABOUT WORKING WITH DIAMOND LIGHT SOURCE?
The community, the opportunity, the constant discovery. People from around the world come together at Diamond Light Source from so many different backgrounds: to run the organisation, build new instruments, create software to help the scientists make better measurements, and to conduct research. It’s valuable to emphasise the diversity of the research that can be done at synchrotrons, from art history to astrophysics to clinical imaging. And then there’s the diversity of scales – sizes, temperatures and pressures – that can be accessed. For example, large turbine blades can be imaged for structural flaws and the structure of a virus or binding in a tiny molecule can be analysed.
EXPLORE A CAREER AT DIAMOND LIGHT SOURCE AND OTHER SYNCHROTRONS
• Diamond Light Source offers school work experience, ‘Year in Industry’ placements, summer placements and PhD studentships: www.diamond.ac.uk/Careers/Students.html
• The work experience is offered to GCSE and A-level students and spans many areas, including communications, data analysis, digital content creation, electronics, health physics, mechanical engineering, project planning and software development: www.diamond.ac.uk/Careers/Students/Schools-Work-Experience.html
• If you’re looking for an apprenticeship, Diamond Light Source has information about possible apprenticeship opportunities at the facility: www.diamond.ac.uk/Careers/Apprenticeships.html
• It is also worth browsing through the ‘Careers’ section of the website to have an idea of the breadth of skills needed at Diamond Light Source: www.diamond.ac.uk/Careers.html
• If you are not based in the UK, visit the websites of synchrotrons near you. Many will provide similar opportunities: lightsources.org/lightsources-of-the-world/
Do you have a question for Jo or a member of the team?
Write it in the comments box below and Jo or a member of the team will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)