The brain is the next frontier
Dr Amanda Brown is a neuroscience researcher based at Johns Hopkins University in the US. She is studying inflammation and neuronal injury in the brain, particularly that caused by HIV-associated disorders. The findings could lead to the development of new and better treatments
TALK LIKE A NEUROSCIENCE RESEARCHER
HIV – a virus that damages the cells in your immune system and weakens your ability to fight everyday infections and disease
HIV-ASSOCIATED NEUROCOGNITIVE DISORDER (HAND) – a syndrome of progressive deterioration of memory, cognition, behaviour and motor function in HIV-infected individuals that can range from mild to severe with extensive immunodeficiency
OSTEOPONTIN – a protein that is implicated in the pathogenesis of a variety of disease states, including several chronic inflammatory diseases
ANTI-RETROVIRAL THERAPY (ART) – any HIV treatment that uses a combination of two or more drugs
INFLAMMATION – the body’s process of fighting against things that harm it, such as infections, injuries and toxins, in an attempt to heal itself
CENTRAL NERVOUS SYSTEM – a system consisting of the brain and spinal cord. It is referred to as ‘central’ because it combines information from the entire body and coordinates activity across the whole organism
MICROGLIA – a type of neuroglia (glial cell) located throughout the brain and spinal cord
MACROPHAGES – specialised cells involved in the detection, phagocytosis and destruction of bacteria and other harmful organisms
PHAGOCYTOSIS – the process by which a cell ingests other cells
HIV (human immunodeficiency virus) is a virus that, over time, damages the cells in your immune system. Eventually, it weakens a patient’s ability to fight everyday infections and disease, which can result in death. There is currently no cure for HIV, but improved understanding has led to better treatments, the result of which has been HIV patients living near-normal lifespans.
Early diagnosis is extremely important in ensuring that HIV patients live long and fruitful lives. However, even at an early stage, HIV is able to enter the brain and cause damage that disrupts the proper neural function of a patient. Dr Amanda Brown is a neuroscience researcher who is working on improving our understanding of the precise mechanisms involved in HIV-associated neurocognitive disorder (HAND). Based at Johns Hopkins University in the US, Amanda started out studying microbiology and immunology, before moving across to neuroscience research.
The starting point for Amanda’s research was to ascertain the significance of HIV entering the brain at an early stage. “Research using different experimental models suggests that the protective and basic functions of the blood-brain-barrier are compromised during infection. In this context, HIV particles and infected immune cells from the blood gain entry into the brain at a higher frequency,” explains Amanda. “The impact of this is significant because the major cell type in which the virus replicates are microglia. Microglia have a relatively long lifespan and are replaced by new ones on the order of months to years. In addition, HIV replication in microglia does not result in their immediate death. This means that HIV-infected microglia can reside in the brain, act as a reservoir and disrupt the proper neural function.”
HOW DOES HAND IMPACT A PATIENT’S LIFE?
Inflammation and neuronal injury to the brain can cause many problems, to differing extents, across patients. The inflammation and injury can be more or less severe in people, but common issues include people having difficulty remembering important tasks, such as taking their medicine (which has knock-on effects that are problematic in their own right). There are also instances where people struggle to focus on a task for any length of time or make decisions. Amanda and other researchers in the field have come to understand the evolving nature of HAND and the role that anxiety, depression and sleep disorders can play.
CAN’T HIV TREATMENTS PREVENT HAND?
Treatments and therapies for HIV patients have come a long way over the past couple of decades. Anti-retroviral therapy (ART) has been shown to be highly effective at remedying many of the issues relating to HIV and extending a patient’s life. However, unfortunately, low-level viral replications and inflammation are still able to persist in patients on ART. “Both clinical and laboratory-based research suggests that not all antiviral treatments penetrate into brain tissue at concentrations needed to completely block HIV replication,” explains Amanda. “While there are excellent treatments that block many steps in the HIV life cycle, as yet there is no drug used in the clinic that can stop the stage where viral DNA is transcribed into RNA (ribonucleic acid), which is when we also know that other tissues in the body – while more accessible to anti-retroviral drugs – can still harbour the virus in a state that resists complete eradication.”
WHAT ROLE DOES OSTEOPONTIN PLAY IN HAND?
Osteopontin (OPN) is a protein that is implicated in the pathogenesis of a variety of disease states, including HIV. One of Amanda’s focuses is understanding more about the role of OPN in HAND – whether it contributes to the problem, is a result of it, or both. It is known that OPN induction is a protective response of host cells to the injuries caused by HIV infection, but there is still more work to be done. “OPN has many functions, one of which is its ability to recruit other immune cells acting as a chemokine or cytokine. OPN expression is elevated over normal levels in the central nervous system of people living with HIV who have moderate to severe cognitive impairment,” says Amanda. “Early on, we thought OPN was contributing to the problem, but all our evidence to date suggests that OPN acts to block the negative impacts of HIV replication and the chronic inflammation associated with it.”
WHAT ELSE IS KNOWN ABOUT OPN AND HOW ARE HYPOTHESES TESTED?
Amanda and the team have been conducting experiments on OPN and have discovered that OPN signalling through specific integrin receptors activates the mTORC (mammalian target of rapamycin) which is linked to many pro-survival, growth stimulating and metabolic pathways. They are also investigating the pathways in the brain that OPN engages to regulate inflammatory signalling. The team began their studies using culture human macrophages, but needed to understand whether their observations were relevant to what people living with HIV experience. They tested their hypotheses using blood, cerebrospinal fluid and post-mortem brain tissue. “There are good rodent models that mimic specific aspects of HIV neuropathogenesis,” explains Amanda. “The ability to conduct such studies in an ethical fashion is important because it is the best way to most accurately study the brain.”
Amanda and her team believe the new research they are performing will help develop understanding of how OPN turns inflammatory signalling up and down – and which proteins are responsible. If this can be ascertained, it could lead to new and better treatments that prevent or minimise the inflammation and neuronal injury that can take place in the brain as a result of HIV.
 DR AMANDA BROWN
DR AMANDA BROWN
Associate Professor, Department of Neurology and Neuroscience, School of Medicine, Johns Hopkins University, Baltimore, Maryland, USA
FIELD OF RESEARCH: Neuroimmunology and Neuroscience
RESEARCH PROJECT: Developing understanding of the role that osteopontin plays in HIV-associated neurocognitive disorder. The findings could lead to new and improved treatments that benefit patients around the world
FUNDER: National Institutes of Neurological Disorders and Stroke, National Institutes of Health and National Institutes of Mental Health (USA)
ABOUT NEUROSCIENCE RESEARCH
Neuroscience is a relatively new discipline that arose just a few decades ago. Amanda took what could be considered an atypical pathway into it, having previously focused on microbiology and immunology, but that only serves to highlight the breadth of neuroscience research and how it links to many other scientific disciplines.
The brain is one of the – if not the – most complex things in the Universe, so it follows that understanding more about it relies on multidisciplinary research, with contributions from engineers, physicists, mathematicians, biologists and immunologists. While Amanda works with HIV at present, her research translates to neurodegenerative disorders and other viruses that affect the brain. Thus, her findings, and those of her colleagues, can have significant impacts across a broad range of disciplines and applications.
WHAT DOES A TYPICAL DAY LOOK LIKE FOR A RESEARCH NEUROSCIENTIST?
One of the most appealing aspects of being a neuroscience researcher is that there is no such thing as a typical day! Amanda begins most days talking to her research staff, as well as students (she has undergraduates working in her laboratory), but she is still engaged in practical research at the bench. “We will soon be ramping up our animal studies, so once that has been set up, we will check the animals every day, maybe collecting blood samples for analysis,” says Amanda. “Sometimes, there will be seminars to present so that we can communicate science to our peers, but also feature guest speakers. Before you know it, the day is over!”
WHAT WILL THE NEXT GENERATION OF RESEARCHERS FACE?
The brain can reasonably be considered the next frontier for science. Understanding has developed significantly in the past few years, but there is still so much we do not know. For Amanda, understanding how we can repair or modify the brain to protect against diseases such as Alzheimer’s will be a key focus. There is also much work to be done on microglia and the central nervous system. “We all need some inflammation, as that is a necessary mechanism to protect us against various threats,” explains Amanda. “But if we cannot properly understand inflammatory signalling, there is always the danger that it could go overboard and represent a threat that causes damage. Understanding how we can modulate inflammation or develop treatments that thwart it is certainly an area of research I expect to develop in the future.”
Reference
https://doi.org/10.33424/FUTURUM171
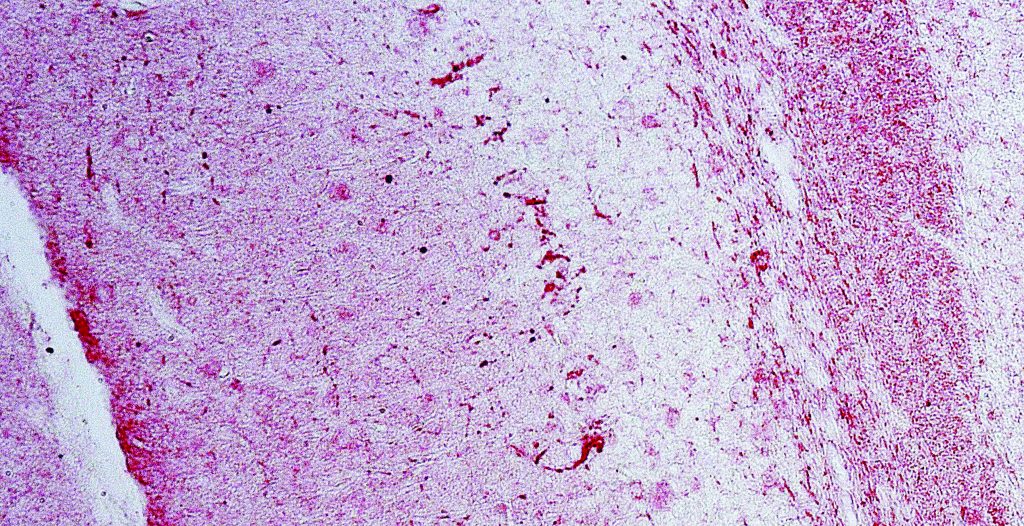
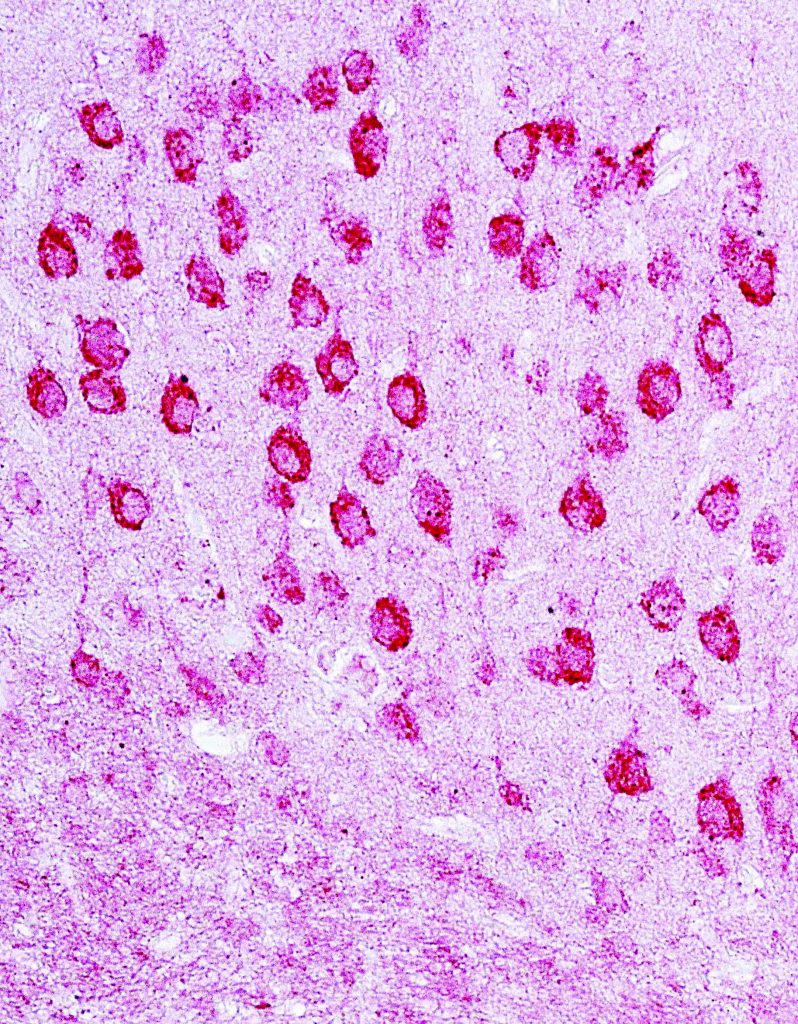
very high resolution. Human monocyte-derived macrophage infected with an engineered HIV virus that expresses the enhanced green fluorescent protein
(EGFP). The uninfected macrophages are labeled with an antibody that detects a surface receptor known as CD81 (blue colour).
HIV – a virus that damages the cells in your immune system and weakens your ability to fight everyday infections and disease
HIV-ASSOCIATED NEUROCOGNITIVE DISORDER (HAND) – a syndrome of progressive deterioration of memory, cognition, behaviour and motor function in HIV-infected individuals that can range from mild to severe with extensive immunodeficiency
OSTEOPONTIN – a protein that is implicated in the pathogenesis of a variety of disease states, including several chronic inflammatory diseases
ANTI-RETROVIRAL THERAPY (ART) – any HIV treatment that uses a combination of two or more drugs
INFLAMMATION – the body’s process of fighting against things that harm it, such as infections, injuries and toxins, in an attempt to heal itself
CENTRAL NERVOUS SYSTEM – a system consisting of the brain and spinal cord. It is referred to as ‘central’ because it combines information from the entire body and coordinates activity across the whole organism
MICROGLIA – a type of neuroglia (glial cell) located throughout the brain and spinal cord
MACROPHAGES – specialised cells involved in the detection, phagocytosis and destruction of bacteria and other harmful organisms
PHAGOCYTOSIS – the process by which a cell ingests other cells
HIV (human immunodeficiency virus) is a virus that, over time, damages the cells in your immune system. Eventually, it weakens a patient’s ability to fight everyday infections and disease, which can result in death. There is currently no cure for HIV, but improved understanding has led to better treatments, the result of which has been HIV patients living near-normal lifespans.
Early diagnosis is extremely important in ensuring that HIV patients live long and fruitful lives. However, even at an early stage, HIV is able to enter the brain and cause damage that disrupts the proper neural function of a patient. Dr Amanda Brown is a neuroscience researcher who is working on improving our understanding of the precise mechanisms involved in HIV-associated neurocognitive disorder (HAND). Based at Johns Hopkins University in the US, Amanda started out studying microbiology and immunology, before moving across to neuroscience research.
The starting point for Amanda’s research was to ascertain the significance of HIV entering the brain at an early stage. “Research using different experimental models suggests that the protective and basic functions of the blood-brain-barrier are compromised during infection. In this context, HIV particles and infected immune cells from the blood gain entry into the brain at a higher frequency,” explains Amanda. “The impact of this is significant because the major cell type in which the virus replicates are microglia. Microglia have a relatively long lifespan and are replaced by new ones on the order of months to years. In addition, HIV replication in microglia does not result in their immediate death. This means that HIV-infected microglia can reside in the brain, act as a reservoir and disrupt the proper neural function.”
HOW DOES HAND IMPACT A PATIENT’S LIFE?
Inflammation and neuronal injury to the brain can cause many problems, to differing extents, across patients. The inflammation and injury can be more or less severe in people, but common issues include people having difficulty remembering important tasks, such as taking their medicine (which has knock-on effects that are problematic in their own right). There are also instances where people struggle to focus on a task for any length of time or make decisions. Amanda and other researchers in the field have come to understand the evolving nature of HAND and the role that anxiety, depression and sleep disorders can play.
CAN’T HIV TREATMENTS PREVENT HAND?
Treatments and therapies for HIV patients have come a long way over the past couple of decades. Anti-retroviral therapy (ART) has been shown to be highly effective at remedying many of the issues relating to HIV and extending a patient’s life. However, unfortunately, low-level viral replications and inflammation are still able to persist in patients on ART. “Both clinical and laboratory-based research suggests that not all antiviral treatments penetrate into brain tissue at concentrations needed to completely block HIV replication,” explains Amanda. “While there are excellent treatments that block many steps in the HIV life cycle, as yet there is no drug used in the clinic that can stop the stage where viral DNA is transcribed into RNA (ribonucleic acid), which is when we also know that other tissues in the body – while more accessible to anti-retroviral drugs – can still harbour the virus in a state that resists complete eradication.”
WHAT ROLE DOES OSTEOPONTIN PLAY IN HAND?
Osteopontin (OPN) is a protein that is implicated in the pathogenesis of a variety of disease states, including HIV. One of Amanda’s focuses is understanding more about the role of OPN in HAND – whether it contributes to the problem, is a result of it, or both. It is known that OPN induction is a protective response of host cells to the injuries caused by HIV infection, but there is still more work to be done. “OPN has many functions, one of which is its ability to recruit other immune cells acting as a chemokine or cytokine. OPN expression is elevated over normal levels in the central nervous system of people living with HIV who have moderate to severe cognitive impairment,” says Amanda. “Early on, we thought OPN was contributing to the problem, but all our evidence to date suggests that OPN acts to block the negative impacts of HIV replication and the chronic inflammation associated with it.”
WHAT ELSE IS KNOWN ABOUT OPN AND HOW ARE HYPOTHESES TESTED?
Amanda and the team have been conducting experiments on OPN and have discovered that OPN signalling through specific integrin receptors activates the mTORC (mammalian target of rapamycin) which is linked to many pro-survival, growth stimulating and metabolic pathways. They are also investigating the pathways in the brain that OPN engages to regulate inflammatory signalling. The team began their studies using culture human macrophages, but needed to understand whether their observations were relevant to what people living with HIV experience. They tested their hypotheses using blood, cerebrospinal fluid and post-mortem brain tissue. “There are good rodent models that mimic specific aspects of HIV neuropathogenesis,” explains Amanda. “The ability to conduct such studies in an ethical fashion is important because it is the best way to most accurately study the brain.”
Amanda and her team believe the new research they are performing will help develop understanding of how OPN turns inflammatory signalling up and down – and which proteins are responsible. If this can be ascertained, it could lead to new and better treatments that prevent or minimise the inflammation and neuronal injury that can take place in the brain as a result of HIV.
 DR AMANDA BROWN
DR AMANDA BROWN
Associate Professor, Department of Neurology and Neuroscience, School of Medicine, Johns Hopkins University, Baltimore, Maryland, USA
FIELD OF RESEARCH: Neuroimmunology and Neuroscience
RESEARCH PROJECT: Developing understanding of the role that osteopontin plays in HIV-associated neurocognitive disorder. The findings could lead to new and improved treatments that benefit patients around the world
FUNDER: National Institutes of Neurological Disorders and Stroke, National Institutes of Health and National Institutes of Mental Health (USA)
ABOUT NEUROSCIENCE RESEARCH
Neuroscience is a relatively new discipline that arose just a few decades ago. Amanda took what could be considered an atypical pathway into it, having previously focused on microbiology and immunology, but that only serves to highlight the breadth of neuroscience research and how it links to many other scientific disciplines.
The brain is one of the – if not the – most complex things in the Universe, so it follows that understanding more about it relies on multidisciplinary research, with contributions from engineers, physicists, mathematicians, biologists and immunologists. While Amanda works with HIV at present, her research translates to neurodegenerative disorders and other viruses that affect the brain. Thus, her findings, and those of her colleagues, can have significant impacts across a broad range of disciplines and applications.
WHAT DOES A TYPICAL DAY LOOK LIKE FOR A RESEARCH NEUROSCIENTIST?
One of the most appealing aspects of being a neuroscience researcher is that there is no such thing as a typical day! Amanda begins most days talking to her research staff, as well as students (she has undergraduates working in her laboratory), but she is still engaged in practical research at the bench. “We will soon be ramping up our animal studies, so once that has been set up, we will check the animals every day, maybe collecting blood samples for analysis,” says Amanda. “Sometimes, there will be seminars to present so that we can communicate science to our peers, but also feature guest speakers. Before you know it, the day is over!”
WHAT WILL THE NEXT GENERATION OF RESEARCHERS FACE?
The brain can reasonably be considered the next frontier for science. Understanding has developed significantly in the past few years, but there is still so much we do not know. For Amanda, understanding how we can repair or modify the brain to protect against diseases such as Alzheimer’s will be a key focus. There is also much work to be done on microglia and the central nervous system. “We all need some inflammation, as that is a necessary mechanism to protect us against various threats,” explains Amanda. “But if we cannot properly understand inflammatory signalling, there is always the danger that it could go overboard and represent a threat that causes damage. Understanding how we can modulate inflammation or develop treatments that thwart it is certainly an area of research I expect to develop in the future.”
EXPLORE A CAREER IN NEUROSCIENCE RESEARCH
• Amanda suggests that budding neuroscientists should look for societies, such as the Society for Neuroscience, to help them understand what is happening in the field.
• The British Neuroscience Association has a wealth of resources that are designed to help people understand the wonders of the brain.
There is also a list of current positions, which should give you an idea of the breadth of possibility in the field.
• According to Payscale.com, the starting salary for a neuroscientist with a master’s degree in the US is $44,000, and the average salary is $81,000.
PATHWAY FROM SCHOOL TO NEUROSCIENCE RESEARCH
Most neuroscientists complete a science-based undergraduate degree followed by a PhD. Some will also complete a master’s degree. As neuroscience combines many scientific disciplines, there are a range of physical and life sciences degrees that can form a starting point to your career, including biochemistry, biology, biomedical sciences, pharmacology and psychology.
HOW DID AMANDA BECOME A NEUROSCIENCE RESEARCHER?
WHAT WERE YOUR INTERESTS AS A YOUNGSTER?
My family moved from a city area to a more rural area which made me fascinated with nature – there were lizards and snakes… we even had tarantulas! With the seasons, the insects and birds around us changed. I was always very observant about nature and noticed the stars at night. Astronomy involves the challenge of math and physics, but I think my curiosity in nature and how things worked set me on my current pathway.
WHO OR WHAT INSPIRED YOU TO BECOME A SCIENTIST?
I always wanted to be an educator, so I found out what I could about teaching and having wonderful teachers myself helped. It was at college that my biology professor talked about research and that process of discovery, and I had the opportunity to do research at a deeper level. During the summers, I went to other universities around the country to do research and learned about how one can make a living doing research. And so that led to graduate school.
That started this pathway of developing as an independent researcher, because it does take time to learn the scientific method, how to ask questions, and how to communicate. Having the self-efficacy and confidence in making observations, developing hypotheses, and understanding what experiments are needed to test it. Research science is not an occupation that is dominant within the African American community that I come from. It became clear that there’s an opportunity – a big gap in having young people like me, or young people in general, to understand what science is, what we do, and that it can be a valuable, enriching career.
WHAT ATTRIBUTES HAVE MADE YOU A SUCCESSFUL SCIENTIST?
Research can take a lot of time and there are so many publications coming out, so it involves a lot of reading. Fortunately, I am passionate about reading and consider it a hobby, so that helps! In fact, I am not sure that people who see academia as just a job could have a successful career. When you are the head of a lab, or you are working in an academic environment, you are trying to improve things and understand what is out there in the field – being passionate about what you do and believing in it is essential.
HOW DO YOU SWITCH OFF FROM YOUR WORK?
I love gardening and cooking, which helps me get out of the lab and into a different headspace. I like to travel and see new places – and I love music and dancing!
WHAT IS YOUR PROUDEST ACHIEVEMENT SO FAR?
That I am still here doing this work. When I started, there were less programmes to encourage women and minorities. Universities weren’t originally designed to welcome a diversity of people – it could feel like a foreign environment, and you question whether you belong. When you get your PhD, you show that you understand how science is done and can apply it to a new problem. You move from school to a professional environment and then you transition to an independent head of lab position. At each of those transitions, you question whether you are good enough. I am now at Johns Hopkins, I’ve secured funding, I’ve got recognition from the institution, my employer and the NIH and I’m an associate professor, looking to be a professor. I never envisioned that I would be in this position. That is why I say my greatest accomplishment is persisting, working hard and getting to where I am now.
AMANDA’S TOP TIPS
01 Take advantage of the fact that you are just starting out and still know relatively little. I often find that as trainees get older, they become more reluctant to reach out to people, so never be afraid to ask questions. Knocking on doors can help you make progress, so bear that in mind.
02 Try and maintain a good support network through friends and family members. There will be times when the research is tough but having friends and family to support you will be a big help.
03 There is nothing wrong with failing; in fact, failing fast is the best. Then you can regroup and try again!
Do you have a question for Amanda?
Write it in the comments box below and Amanda will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)