Unexpected ways that paint prevents corrosion
Our modern lifestyles depend on the material objects around us remaining strong and free from damage. Protective paints and coatings are effective at preventing corrosion damage, but how they do this – and how these mechanisms can be optimised – are less well understood. The sustainable coatings by rational design (SusCoRD) project connects materials science academics with commercial scientists to tackle these questions
TALK LIKE A MATERIALS SCIENTIST
ACTIVE INGREDIENT — a component of a material that is responsible for a particular effect or property
CORROSION — the gradual destruction of a material (usually a metal) and its properties (like strength) by chemical or electrochemical reaction with its environment
THERMODYNAMICALLY STABLE — the state of a material or system being in chemical balance with its environment
ELECTROCHEMICAL — a reaction that involves both electrical and chemical changes
EMPIRICAL DESIGN — an approach based on evidence and observation that does not try to fully understand how things work (e.g., Kepler’s Laws that describe how the planets orbit the sun – predating Newton’s laws of gravity)
OXIDISED — combine chemically with oxygen
NANOCOMPOSITE — a composite combines several different materials to optimise its useful properties; in a nanocomposite, some components are at the nanoscale (less than 1 micron in size)
OXIDATION — the loss of electrons during a chemical reaction by a molecule, atom or ion. See BBC Bitesize for a more detailed explanation: www.bbc.co.uk/bitesize/guides/z7rswty/revision/1
PIGMENT — a substance that gives paint a useful function like colour, hardness, and resistance to weathering and corrosion
POLYMER — a substance (such as plastic) made up of many similar molecular units bonded together
RATIONAL DESIGN — an approach that makes predictions based on understanding and modelling how things work (e.g., Einstein’s Theory of General Relativity that explains gravity)
From tower blocks to cargo ships, suspension bridges to wind turbines, modern society depends on metal structures that resist corrosion and remain dependable for years. Corrosion-protective paints make this possible, but there are gaps in our knowledge that prevent us from explaining how exactly such paints work. Professor Stuart Lyon and Dr Yanwen Liu, from the University of Manchester, and Dr Andy Parnell, from the University of Sheffield, are part of a research team that has partnered with Anglo-Dutch coatings company AkzoNobel to fill these gaps using a wide range of innovative scientific techniques.
PAINT AND CORROSION
“Aside from its decorative aspects, paint can have many functional qualities,” says Stuart. “In the SusCoRD partnership, we’re focusing specifically on coatings that protect metal surfaces from corrosion.” Paints typically contain a complex mix of ingredients, each with its own purpose. These can include binders that help “glue” the paint together, pigments that give the paint its colour and other useful properties, solvents that help the ingredients mix properly and many others. “The binder is generally a cross-linking organic polymer, and the functional pigments are often on the nanoscale,” says Yanwen. “Paint is therefore a sophisticated, complex and functional polymer composite material.” The team aims to understand how the nano-scale properties of paints define their overall function.
Corrosion is a process in which a material reacts with its environment in a way that leads to a loss of function, typically the material’s strength. “Interactions with water molecules can cause metal to corrode, turning its atoms into ions that release electrons,” explains Andy. “These electrons enter a second chemical reaction, usually involving the consumption of oxygen. Corrosion is, therefore, an electrochemical reaction, because both chemical and electrical processes are involved.”
PROTECTING AGAINST CORROSION
Corrosion occurs because the thermodynamically stable ‘natural’ state of a metal is to be oxidised. Metal ores are mined in their oxidised state, and energy is added in the refining process to reduce the ores to the metal. Over time, however, metals will corrode naturally and return to their most stable, oxidised form, losing their useful properties in the process. “Because corrosion is an electrochemical reaction and needs water and oxygen, it can be controlled by removing these reactants and/or by stopping the flow of current,” says Stuart. “It is possible to remove oxygen from closed systems, such as in central heating or engine-cooling systems, but for most systems open to the air, another protection method is needed.”
There are three main theories about how corrosion-protective paints work:
- The paint acts as a physical barrier between the metal and any oxygen or water in the environment.
- The paint acts as an electrical insulator, slowing the flow of current that drives the electrochemical reaction of corrosion.
- There are active ingredients within paint that gather at sites where corrosion has begun and inhibit further corrosion.
It is likely that all three of these processes play a part in corrosion protection. The team is interested in discovering which are most significant and how the processes can be optimised to produce the most corrosion-protective paint possible.
TESTING CORROSION-PROTECTIVE PAINTS
Until recently, most research depended on empirical design, a scientific philosophy that emphasises the importance of evidence to build conclusions. “This ‘trial and error’ approach uses existing knowledge and experience to make a first approximation to what might work and then tests this approximation, explains Yanwen. However, this becomes impractical when testing corrosion-protective paints because there is a need to prove that these paints are effective for decades. “We develop ‘accelerated’ tests in the lab, which mimic the effects of decades-long exposure to the environment in a much shorter time,” says Andy. “However, we have to accept that these aren’t completely realistic and need to find ways to account for that.”
This means that developments in the field can take time, especially if there is a need to develop completely new materials or systems where there is no existing knowledge base to draw on. “For instance, it took 20 years to find an effective replacement to lead-based paints to protect steel from corrosion,” says Stuart. “We are still trying to find an optimum substitute for chromate, currently used in paints, to suppress corrosion of aluminium aircraft.” While they are successful ingredients for corrosion protection, lead- and chromate-based paints present significant hazards to human health.
SOLUTIONS THROUGH RATIONAL DESIGN
Fortunately, the rise of molecular science and computer technology is helping address this time problem. As an alternative to empirical design, ‘rational design’ aims to discover how substances work at a fundamental level and then model potential combinations, rather than just observing their effects through trial and error. Once ingredients’ properties are understood – including how they interact with one another – this information is plugged into computer models, which can then simulate how different combinations of ingredients behave in different environments over time. “This approach helps reduce the number of formulations to trial in the real world because the computer models inform us quickly about many formulations that definitely won’t work,” says Yanwen. “The models also help suggest changes to formulations that may use better, cheaper or more environmentally sustainable ingredients without affecting performance.”
Reference
https://doi.org/10.33424/FUTURUM276
While the primary roles of the individual ingredients in paints are well-understood, the behaviour of the ingredients when combined is what these models aim to test. “These ingredients interact in ways that are currently not predictable,” says Andy. “Aside from providing colour, we know that certain types of pigment provide corrosion protection in some manner. However, they have many different properties and interactions that affect the level of protection they convey.”
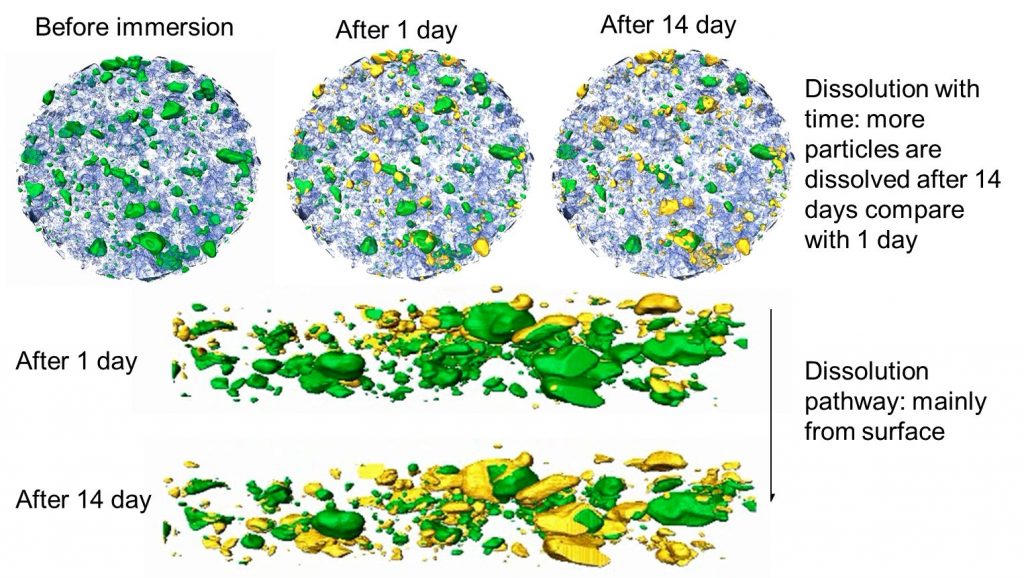
The team’s research suggests that the third theory of active ingredients gathering at sites where corrosion has begun plays a significant role. “The pigments dissolve in the presence of water, and their active ingredients migrate to the corrosion sites and inhibit the reaction,” says Stuart. “This process is influenced by the shape, size and distribution of pigment particles, as well as their interactions with other pigments and solvents, method of application, environmental conditions and so on.”
TESTING METHODS
Understanding how all these materials behave and interact at a fundamental level is no easy task. “We need to study every aspect of the coating in isolation and when attached to metal,” says Yanwen. “This is difficult because analysis of all the different components in the coatings requires different techniques and tools.” In addition, the team has to find ways to examine exactly what happens where the metal meets the paint, where corrosion begins, without disrupting the reactions taking place there.
Fortunately, the team has several high-tech analytical methods at its disposal, from the fields of materials science, surface science and electrochemistry. “We use high-resolution analytical electron microscopy, X-ray tomography and atomic force microscopy to directly observe the materials,” says Andy. “Ellipsometry studies how the paint interacts with surfaces, while X-ray and neutron scattering examine how water moves within the substance.” Several other microscopy techniques help study the electrochemical changes taking place.
Computing is vital throughout. “Mathematical models help us understand how corrosive and anti-corrosive substances behave,” says Stuart. “In addition, AkzoNobel has many years of corrosion test data for their products, which we’re supplying to machine learning algorithms to identify how complex combinations of factors relate to performance.”
REVELATIONS
The team has made considerable progress in discovering what works and what does not in terms of corrosion protection. While the first of the three theories outlined earlier may seem the most ‘obvious’, there is mounting evidence that it plays only a minor role. “It has been known for some time that paint doesn’t effectively block the migration of water and oxygen, which is why decorative wall paint is useless for preventing corrosion of steel,” says Yanwen. “So, this method is not how paint works to protect against corrosion.”
There is more evidence for electrical resistance, the second theory, as an effective corrosion blocker. “However, our research suggests this resistance does not come from the paint alone, but from the paint-metal interface, which is where the corrosion starts,” says Stuart. “Our current focus is on testing this hypothesis.” This points toward a combination of the second and third theories as defining corrosion protection.
The team has made other insightful discoveries. “It might be assumed that a greater concentration of anti-corrosion pigment would lead to a more effective coating,” says Andy. “However, we’ve found that high concentrations leach out of the paint faster, giving the coating a shorter lifetime. Based on these findings, we’re developing a computer model to predict the optimum amount of active ingredient.”
ABOUT MATERIALS SCIENCE
Materials science involves the design, study and discovery of new materials. This broad scope means that it interacts with many other STEM disciplines. “Materials science provides the link between understanding a material’s microstructure at the nanoscale, to discovering how these properties affect a material’s function at the macroscale,” says Andy. “Understanding these properties involves using tools such as imaging, characterisation and simulation. Additionally, materials scientists may use this understanding to create new useful materials through synthesis, processing, manufacture or modelling.”
WHY IS MATERIALS SCIENCE IMPORTANT?
“Materials are central to the socio-economic well-being of the country,” Stuart explains. “Materials scientists and engineers help to develop the materials required for new products, improve and lower the cost of manufacturing routes and enhance the performance of existing materials. They consider the environmental impact and sustainability of their products, for example, by replacing materials that rely on scarce elements or are hazardous to health. They discover how to optimise the selection of materials and create sophisticated models and databases from which properties can be predicted.”
HOW DO MATERIALS SCIENTISTS FIT INTO THE SUSCORD PARTNERSHIP?
“The problem is too complex for a single expert to cover, so a multidisciplinary approach is essential,” says Yanwen. “Our academic research team consists of chemists, physicists, materials scientists, microscopists, electrochemists, mathematicians, computational modellers and corrosion experts. This is supported from the AkzoNobel side by paint chemists, polymer physicists, formulation chemists and experts in testing and evaluation and in industrial paint making. Finally, and probably most importantly, is Dr Jane Deakin, our project manager, who supports the team, reminds us of our overall aims and objectives, organises meetings and generally keeps us on our toes.”
WHY SHOULD YOUNG PEOPLE CONSIDER A CAREER IN MATERIALS SCIENCE?
“Materials scientists or engineers are always in high demand and are relatively well rewarded,” says Stuart. “They may be employed in many sectors, with careers in manufacturing, research, product development, consultancy or education. Areas that are in need of materials scientists include advanced transport systems, healthcare, energy generation, environmental protection and electronics.”
• The communities of all the universities participating in the SusCoRD project are strong in materials science and manufacturing. Manchester has the largest and most diverse materials department in the UK, while Sheffield and Liverpool have strong manufacturing histories. During the annual British Science Week, these departments engage in public outreach, which can be a good opportunity to learn more.
• Nuffield Research Placements are a valuable way to learn more about STEM, including materials science, through gaining research skills and enhancing university applications: www.nuffieldfoundation.org/students-teachers/nuffield-research-placements
• The Institute of Materials, Minerals and Mining (IoM3) provides a wide range of relevant outreach activities and resources for school students: www.iom3.org/careers-learning/schools-outreach.html
• According to Payscale, salaries for early career materials specialists in the UK are around £32,000. This is the higher end of engineering and science professions and increases significantly as people progress to more senior roles.
For a standard entry into materials science, the researchers recommend taking A-Levels or equivalents in physics, chemistry and maths, as well as biology if possible. “As materials science is not taught specifically in schools, university courses start at the basics,” says Stuart. “Most universities also offer entry routes via BTEC level 3 in maths and science as well as access and foundation routes to undergraduate study.”
Aside from university, there are also BTEC courses available for materials science, which can lead to opportunities within the manufacturing industry where a strong technical knowledge base is essential.
MEET THE TEAM

PROFESSOR STUART LYON
AKZONOBEL PROFESSOR OF CORROSION CONTROL, DEPARTMENT OF MATERIALS, UNIVERSITY OF MANCHESTER, UK
FIELD OF RESEARCH: MATERIALS SCIENCE, ELECTROCHEMISTRY AND CORROSION PROTECTION
As a child, I was interested in everything around me, from the natural world to functional man-made objects. I was always taking things apart to see how they worked. I remember I wanted a wristwatch so badly at the age of nine and had to promise never to take it apart before my parents would buy it! My father was a mechanical engineer and often called me into the garage to help him maintain his car with the help of my “small fingers”.
Professors have two main roles. The first is to teach what we know to students. The second is to undertake research to make new discoveries, and then teach these too. Research does not just involve time in the lab, but also securing finances by pitching ideas to companies or government funders, recruiting and supporting researchers, and navigating the publication and dissemination of results.
I graduated with a degree in metallurgy and materials science and then continued with a PhD. Despite a severe economic recession when I completed this, I was fortunate to secure a post-doctoral position working in corrosion prevention, and then started climbing the academic ladder over the following 25 years.
I don’t undertake any research myself – this is done by postgraduate students or more experienced post-doctoral researchers. As a principal investigator, I lead and manage a team of investigators and researchers who undertake the work. I ensure that individual projects are going well and milestones are being met. I also spend time with my own researchers who I supervise to discuss how things are going.
Academia can sometimes be lonely, but the remarkable thing about SusCoRD is that we have built a genuine partnership between a large multinational enterprise, AkzoNobel, and three UK universities across four disciplines. It’s really exciting to work with such a talented and knowledgeable group of people.
The most frustrating aspect of research is usually its slow place. We’re all desperate to prove our great ideas, but time is needed to set up experiments correctly and develop the skills needed. You must have faith in your ideas and continue trying.
I’m fascinated by materials and how they can be scientifically designed and then engineered into products with almost magical properties. The fine-scale structure of a material generally controls its property and investigating this to the almost atomic level is so intriguing.
I enjoy physical activities such as hiking, walking my dog and cycling. I also love upgrading my house – for instance, I installed a new bathroom last year. And, as you might expect, I always do the painting in my house!

DR YANWEN LIU
RESEARCH FELLOW, DEPARTMENT OF MATERIALS, UNIVERSITY OF MANCHESTER, UK
FIELD OF RESEARCH: CORROSION PROTECTION OF METAL SUBSTRATES BY COATINGS AND INHIBITORS
When I was young, I was very interested in chemistry. I was also keen to understand how things gradually decay over time, such as metal fences. Later, I understood that what I was watching was the corrosion of the metal beneath the paint.
I obtained a degree in petrochemical engineering and worked in the industry for a while, developing lubricants and corrosion-inhibiting oils, before undertaking a PhD at the University of Manchester. During my PhD, I investigated how to slow the corrosion of aerospace aluminium alloys by uncovering the mechanisms at play that defined the functions of various pigments within coatings.
After I completed my PhD, I continued working in the same area, beginning work on projects sponsored by AkzoNobel in 2012, initially to investigate alternatives to toxic chromate-based paints in the architecture and aircraft industry. This eventually led to me becoming a coinvestigator on the SusCoRD project.
My co-investigator role involves the application of advanced analytical microscopy and spectroscopy to understand the physical and chemical degradation processes of metals and coatings. I also use X-ray tomography for the analysis of degradation and leaching pathways.
The project is providing an invaluable way to understand why coatings fail with time, and how to prolong the life expectancy of coatings to protect the metal for as long as possible. The global cost of corrosion is huge, so finding ways these costs could be significantly reduced is rewarding. Moreover, our research is uncovering more environmentally friendly coatings, which will be necessary for a sustainable future.
I find the most challenging part to be balancing the scientific process alongside industry needs. The former benefits from knowledge sharing and lengthy investigation, while the latter requires confidentiality and rapid progress. However, through effective communication with our industry counterparts, our work becomes effective and efficient.
We are able to publish our findings not only from lab observations and results but also from coatings used in the real world in real-time, which gives a real understanding of how coatings actually degrade, compared to simulations and models. This data is very precious for scientific communities. I love to relax by reading at home or going for walks in the countryside.

DR ANDREW PARNELL
RESEARCH FELLOW, DEPARTMENT OF PHYSICS AND ASTRONOMY, UNIVERSITY OF SHEFFIELD, UK
FIELD OF RESEARCH: MATERIALS PHYSICS
I’ve always been adventurous and loved exploring the outdoors on my mountain bike when I was younger. I also had a fascination with technology and spent hours tinkering with computers and radios. As a teenager, I had a strong interest in amateur radio, and a friend and I used to repurpose radio equipment from the police and make our own broadcasts. I became a member of an amateur radio club that let me connect with and learn from many amazing and experienced members. I have a powerful memory of one of the members demonstrating using a laser as a potential communication method, by spraying aerosol in the dark and lighting the room with the laser.
Having studied chemistry, physics and maths at A-level, I took a degree in physics, which made me aware of the many applications of science, including an experimental project on squeezed light that went on to be published. After my degree, I was fortunate to be accepted for a master’s course in nanoscale science and technology, where I learnt everything from self-assembly to organic electronics. This gave me a great generalist skillset, and one project, in particular, led me to undertake a PhD on responsive polyelectrolyte surface grafted brushes. I then went back to the Department of Physics at the University of Sheffield and have since been promoted to a lecturer. My projects have often involved the industry and I’ve worked with talented individuals from many multinational companies.
I work on the surfaces and interfaces work package, which aims to understand how protective coatings work by examining the paint-metal interface in detail. There’s no such thing as a typical day, which helps make my career fun! I help plan, run, troubleshoot and discuss the results that we get.
The return to the lab and seeing my colleagues again, after the worst of the COVID-19 pandemic had passed. The pandemic was a difficult moment to get through.
Learning new experimental techniques can be challenging as it involves reading and re-reading textbooks and academic papers to best understand the possibilities and pitfalls of new techniques. The variety and breadth of materials physics is vast, covering everything from paints and coatings to organic solar cells. This makes the science that I do relevant to a whole array of different arenas, which is immensely rewarding. I love cycling and walking in the Peak District, as well as spending time with my wife and daughter.
RESEARCHERS’ TOP TIPS FOR STUDENTS
01 The world needs scientists for every aspect of society.
Keep your curiosity and explore the world, with your eyes or with microscopes!
02 There are no dumb questions, so don’t be afraid to ask.
Whether about scientific processes or how people got to where they wanted to be.
Do you have a question for the SusCoRD team?
Write it in the comments box below and the SusCoRD team will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)













Fantastic article on the unexpected ways paint helps prevent corrosion! 🌟 It’s amazing how a simple layer of paint can offer such robust protection against the elements. For those tackling corrosion issues, working with professional painters can ensure that you’re using the right type of paint and application techniques for optimal protection. Their expertise can help you choose the best products and methods to extend the lifespan of your surfaces and keep them looking great. Thanks for the informative read!
This was a fascinating read! I never realized how many unexpected benefits paint has when it comes to preventing corrosion. It’s amazing how something as simple as a coat of paint can save so much in maintenance costs and prolong the life of structures. Thanks for sharing these insights!
This was such an eye-opening read! I never realized how much paint can contribute to preventing corrosion beyond just aesthetics. The scientific explanations really helped me understand the process better. I’m definitely reconsidering the importance of choosing the right paint for my projects now!