Using nanotechnology to overcome the adhesion of the bacterial pathogen Staphylococcus Aureus
Professor Yves Dufrêne is a researcher in nanobiophysics with an interest in methicillin-resistant Staphylococcus Aureus (MRSA) bacterial strains. Based at the Louvain Institute of Biomolecular Science and Technology in Belgium, his work focuses on finding new means of thwarting these pathogens and their ability to stick to medical devices and cause life-threatening infections
Methicillin-resistant Staphylococcus aureus (MRSA) strains are pathogenic bacteria that have been shown to have a high level of antibiotic resistance. This is of enormous concern because novel antibiotics are extremely difficult to develop; the last antibiotic class that was successfully introduced as a treatment was discovered years ago. So, when bacteria are shown to be resistant to existing antibiotics, it is a huge cause of alarm, as finding a means of treating patients and saving lives becomes much more difficult.
MRSA is an important member of an emerging class of superbugs causing hospital-acquired (nosocomial) infections that clinicians around the world have expressed concern about. A major problem is that MRSA infections involve the formation of adhering multicellular communities, called biofilms, on tissues and implanted devices such as central venous catheters and prosthetic joints. Overall, bacterial biofilms are estimated to be involved in more than 65% of nosocomial infections! Biofilm related MRSA infections are very difficult to fight, because bacterial cells are protected from host defences and are resistant to many antibiotics. Ultimately, when this pathogen enters the bloodstream, it can trigger severe infections, including endocarditis and sepsis, which can rapidly lead to tissue damage, organ failure and death.
It is with all of these concerns in mind that Professor Yves Dufrêne, based at the Louvain Institute of Biomolecular Science and Technology, UCLouvain, Belgium, has established a project funded by the ERC (European Research Council) that is aiming to understand and prevent adhesion and biofilm formation by these bugs. The findings from this project may pave the way to the development of novel anti-adhesion therapies, complementing antibiotic treatments, therefore contributing to saving the lives of countless patients and creating healthier and cleaner environments inside hospitals.
WHAT ARE THE MAIN QUESTIONS THAT YVES IS TRYING TO ANSWER?
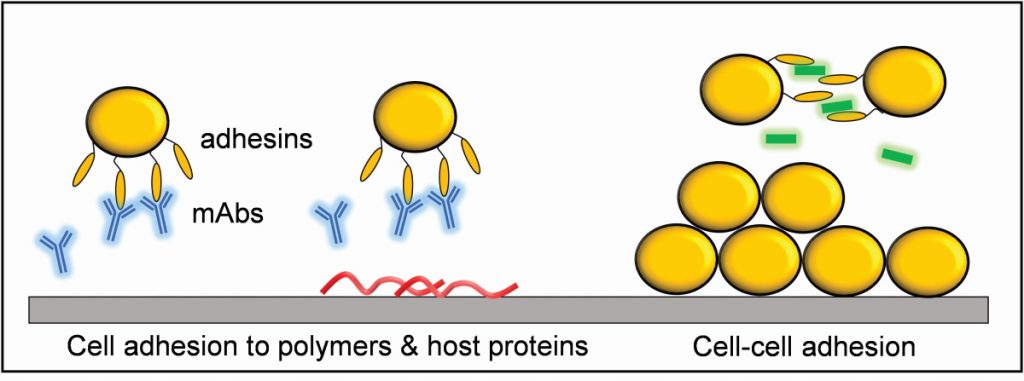
One of the starting points for Yves’ research is to understand how MRSA manages to attach so strongly to medical devices and host tissues, using a variety of adhesion proteins (called adhesins) that decorate the bacterial cell surface. “We have been attempting to understand the molecular mechanisms that drive the specific adhesion of Staphylococcus species to human proteins, such as fibronectin and fibrinogen, which often coat implanted devices,” explains Yves. “In addition, we very much want to identify new molecules (peptides, antibodies) that specifically block this adhesion.”
WHAT TECHNIQUES HAS YVES’ TEAM DEVELOPED?
Over the past 20 years, Yves and his research group have been working on developing atomic force microscopy (AFM) techniques to study a broad range of microbiological aspects, including the structural, adhesive and mechanical properties of the surfaces of microbes. “When it comes to bacterial adhesion, in particular, we have established two complementary approaches to study the interactions between the bacterial adhesin proteins and the human proteins that they bind to. In the first approach, we place a single living S. aureus bacterium on an AFM probe and then bring that cell probe in contact with a substrate on which we have grafted a human host protein. Retracting the cell away from the surface allows us to quantify the physical strength whereby the adhesin binds the human protein,” says Yves. “In the second approach, we graft the human protein onto the AFM probe and then bring it in contact with a single S. aureus cell. This method allows us to study the strength and dynamics of single adhesin-ligand interactions and to map the locations of single adhesins on the bacterial cell surface.”
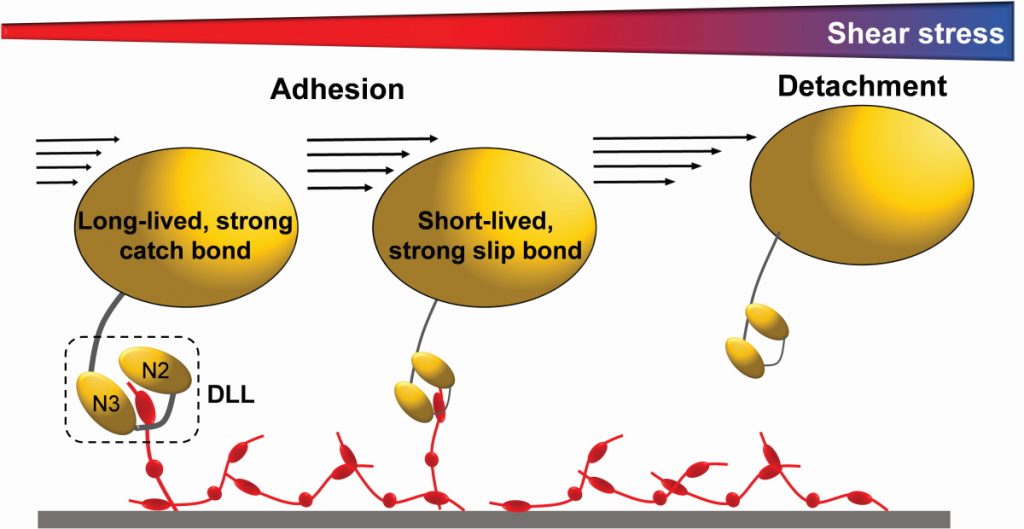
Recently, the team has discovered that some staphylococcal adhesins have the ability to bind their human target proteins with very strong forces and that the lifetime of these bonds grows when they are subjected to increasing physical stress. These so-called catch bonds represent an unusual and counterintuitive mechanism to enhance bacterial adhesion under mechanical stresses, such as those associated with the blood flow, scraping or epithelial turnover.
ANALYSING NANOSCALE SURFACE ARCHITECTURE
AFM images are made by scanning a biological sample like a bacterial cell with an extremely sharp AFM probe (or tip) to produce images that detail the surface topography of the cell with nanometre resolution in physiological aqueous conditions. AFM is a super-resolution imaging technique meaning that the team can produce extremely detailed images of the three-dimensional surface topography of living microbial cells that show much smaller features than what is achievable by most light microscopy techniques. An exciting recent advancement is called multiparametric AFM imaging, where the researchers produce images which show the surface topography, as well as the adhesive and mechanical properties of a bacterium.
WHAT HAS YVES’ RESEARCH REVEALED?
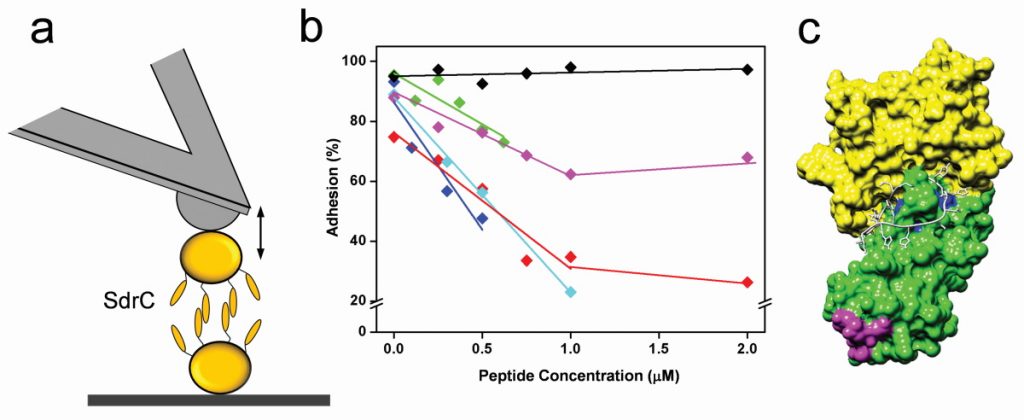
The team has made many exciting discoveries in recent years. “We were the first to find that certain staphylococcal adhesins specifically bind human proteins with extreme mechano stability: the bonds they form are as strong as the covalent bonds that keep the amino acids together in a protein’s polypeptide backbone,” explains Yves. “This is truly unique for a receptor-ligand interaction and critically underlies the ability of staphylococci to form mechanically stable biofilms. As for anti-adhesion therapy, they have demonstrated how special peptides and antibodies can efficiently block the specific binding of adhesins to their targets. This finding shows promise in the development of anti-adhesion agents targeting staphylococcal infections.”
WHAT ARE THE NEXT STEPS?
As you might expect, the team is extremely excited about their recent discovery of the first staphylococcal adhesin that exhibits a catch-bond behaviour. The intention is to expand that knowledge and identify whether such an unusual binding mechanism is used by other staphylococcal adhesins. They are also screening for new peptides and antibodies that could prevent adhesion and biofilm formation, which, if successful, could offer exciting potential for fighting MRSA infections and improving patient outcomes around the world.
 PROFESSOR YVES DUFRÊNE
PROFESSOR YVES DUFRÊNE
Louvain Institute of Biomolecular Science and Technology, UCLouvain, Belgium
FIELD OF RESEARCH: Nanosciences, Biophysics, Microbiology
RESEARCH PROJECT: Yves’ research is focused on pushing the limits of nanotechniques beyond the state-of-the-art to establish them as innovative platforms to understand how pathogens such as MRSA use their surface adhesins to guide cell adhesion and trigger infections, and to develop anti-adhesion strategies for treating biofilm-infections.
FUNDERS: European Research Council (ERC)
NANOTECH MEETS MICROBIOLOGY
Given that microbiology is the study of living organisms that are too small to be visible to the naked eye, it is a field that was always going to benefit from the development of new microscopy techniques enabling researchers to observe microorganisms such as bacteria, viruses and fungi. It was therefore enormously beneficial to microbiologists when the field of nanotechnology began to emerge. In the 1980s, many significant technological developments were made, beginning with the scanning tunnelling microscope (STM) which enabled scientists to observe and manipulate individual atoms (the inventors of STM were later awarded a Nobel Prize). In the mid-1980s, this was followed by the development of atomic force microscopy (AFM), in which a very sharp probe (tip) touches the sample surface with ultra-small forces while raster scanning to obtain topographic images with a resolution of only a few nanometres. AFM is also an ultrasensitive force measuring technique, an approach known as AFM force spectroscopy. Here, force-distance curves are obtained by bringing the probe against the sample and then retracting it while monitoring the force. Attaching specific bioligands to the probe makes it possible to measure the localisation, strength and dynamics of single receptor-ligand interactions, such as staphylococcal adhesin-host protein bonds.
HAVE THERE BEEN ANY EUREKA MOMENTS IN YVES’ RESEARCH?
Of course! In fact, there are probably too many to mention here, but there was the discovery of the ultra-stable adhesion-host protein interactions; the finding of anti-adhesion molecules which could one day help complement antibiotics to treat multi-resistant superbugs; and the first evidence of a catch-bond between an adhesin on a living staphylococcal cell and its target protein. But the findings do not stop there! “For instance, we have done some very exciting work on yeasts, where we have unravelled the force-interaction that plays a role in mating: we now understand yeast sexuality better!” says Yves. “AFM technology is continuously improving, but recent advances in microbiology research include the improvement of imaging speeds, the possibility to do multiparametric imaging (images of topography, adhesion and stiffness generated simultaneously on live cells) and a new modality called fluidic force microscopy where microchanneled probes with micro- or nanosized apertures allow for fast manipulation and analysis of single cells.”
Clearly, this is a field ripe for innovation and new discoveries, so if you are interested in making several significant contributions to a particular branch of molecular and cellular biology, this could be the one for you!
WHAT DOES YVES FIND MOST REWARDING ABOUT HIS WORK?
Yves’ work is truly multidisciplinary, relying strongly on collaborations with microbiologists around the world, which provide clinical strains and bacterial mutants altered in specific adhesins, but also complement AFM data with results from traditional bioassays. It is rewarding to know his team’s work will likely have positive scientific, societal and economic impacts. On a more personal level, Yves is able to work closely with early career researchers who are highly motivated, and to help them achieve their ambitions and develop their skills as scientists. In particular, his former PhD student, David Alsteens, is now a professor at UCLouvain. They share the same instruments and belong to the same nanobio group, but David’s research, also funded by the ERC, mainly focuses on viruses.
Reference
https://doi.org/10.33424/FUTURUM126
Methicillin-resistant Staphylococcus aureus (MRSA) strains are pathogenic bacteria that have been shown to have a high level of antibiotic resistance. This is of enormous concern because novel antibiotics are extremely difficult to develop; the last antibiotic class that was successfully introduced as a treatment was discovered years ago. So, when bacteria are shown to be resistant to existing antibiotics, it is a huge cause of alarm, as finding a means of treating patients and saving lives becomes much more difficult.
MRSA is an important member of an emerging class of superbugs causing hospital-acquired (nosocomial) infections that clinicians around the world have expressed concern about. A major problem is that MRSA infections involve the formation of adhering multicellular communities, called biofilms, on tissues and implanted devices such as central venous catheters and prosthetic joints. Overall, bacterial biofilms are estimated to be involved in more than 65% of nosocomial infections! Biofilm related MRSA infections are very difficult to fight, because bacterial cells are protected from host defences and are resistant to many antibiotics. Ultimately, when this pathogen enters the bloodstream, it can trigger severe infections, including endocarditis and sepsis, which can rapidly lead to tissue damage, organ failure and death.
It is with all of these concerns in mind that Professor Yves Dufrêne, based at the Louvain Institute of Biomolecular Science and Technology, UCLouvain, Belgium, has established a project funded by the ERC (European Research Council) that is aiming to understand and prevent adhesion and biofilm formation by these bugs. The findings from this project may pave the way to the development of novel anti-adhesion therapies, complementing antibiotic treatments, therefore contributing to saving the lives of countless patients and creating healthier and cleaner environments inside hospitals.
WHAT ARE THE MAIN QUESTIONS THAT YVES IS TRYING TO ANSWER?
One of the starting points for Yves’ research is to understand how MRSA manages to attach so strongly to medical devices and host tissues, using a variety of adhesion proteins (called adhesins) that decorate the bacterial cell surface. “We have been attempting to understand the molecular mechanisms that drive the specific adhesion of Staphylococcus species to human proteins, such as fibronectin and fibrinogen, which often coat implanted devices,” explains Yves. “In addition, we very much want to identify new molecules (peptides, antibodies) that specifically block this adhesion.”
WHAT TECHNIQUES HAS YVES’ TEAM DEVELOPED?
Over the past 20 years, Yves and his research group have been working on developing atomic force microscopy (AFM) techniques to study a broad range of microbiological aspects, including the structural, adhesive and mechanical properties of the surfaces of microbes. “When it comes to bacterial adhesion, in particular, we have established two complementary approaches to study the interactions between the bacterial adhesin proteins and the human proteins that they bind to. In the first approach, we place a single living S. aureus bacterium on an AFM probe and then bring that cell probe in contact with a substrate on which we have grafted a human host protein. Retracting the cell away from the surface allows us to quantify the physical strength whereby the adhesin binds the human protein,” says Yves. “In the second approach, we graft the human protein onto the AFM probe and then bring it in contact with a single S. aureus cell. This method allows us to study the strength and dynamics of single adhesin-ligand interactions and to map the locations of single adhesins on the bacterial cell surface.”
Recently, the team has discovered that some staphylococcal adhesins have the ability to bind their human target proteins with very strong forces and that the lifetime of these bonds grows when they are subjected to increasing physical stress. These so-called catch bonds represent an unusual and counterintuitive mechanism to enhance bacterial adhesion under mechanical stresses, such as those associated with the blood flow, scraping or epithelial turnover.
ANALYSING NANOSCALE SURFACE ARCHITECTURE
AFM images are made by scanning a biological sample like a bacterial cell with an extremely sharp AFM probe (or tip) to produce images that detail the surface topography of the cell with nanometre resolution in physiological aqueous conditions. AFM is a super-resolution imaging technique meaning that the team can produce extremely detailed images of the three-dimensional surface topography of living microbial cells that show much smaller features than what is achievable by most light microscopy techniques. An exciting recent advancement is called multiparametric AFM imaging, where the researchers produce images which show the surface topography, as well as the adhesive and mechanical properties of a bacterium.
WHAT HAS YVES’ RESEARCH REVEALED?
The team has made many exciting discoveries in recent years. “We were the first to find that certain staphylococcal adhesins specifically bind human proteins with extreme mechano stability: the bonds they form are as strong as the covalent bonds that keep the amino acids together in a protein’s polypeptide backbone,” explains Yves. “This is truly unique for a receptor-ligand interaction and critically underlies the ability of staphylococci to form mechanically stable biofilms. As for anti-adhesion therapy, they have demonstrated how special peptides and antibodies can efficiently block the specific binding of adhesins to their targets. This finding shows promise in the development of anti-adhesion agents targeting staphylococcal infections.”
WHAT ARE THE NEXT STEPS?
As you might expect, the team is extremely excited about their recent discovery of the first staphylococcal adhesin that exhibits a catch-bond behaviour. The intention is to expand that knowledge and identify whether such an unusual binding mechanism is used by other staphylococcal adhesins. They are also screening for new peptides and antibodies that could prevent adhesion and biofilm formation, which, if successful, could offer exciting potential for fighting MRSA infections and improving patient outcomes around the world.
 PROFESSOR YVES DUFRÊNE
PROFESSOR YVES DUFRÊNE
Louvain Institute of Biomolecular Science and Technology, UCLouvain, Belgium
FIELD OF RESEARCH: Nanosciences, Biophysics, Microbiology
RESEARCH PROJECT: Yves’ research is focused on pushing the limits of nanotechniques beyond the state-of-the-art to establish them as innovative platforms to understand how pathogens such as MRSA use their surface adhesins to guide cell adhesion and trigger infections, and to develop anti-adhesion strategies for treating biofilm-infections.
FUNDERS: European Research Council (ERC)
NANOTECH MEETS MICROBIOLOGY
Given that microbiology is the study of living organisms that are too small to be visible to the naked eye, it is a field that was always going to benefit from the development of new microscopy techniques enabling researchers to observe microorganisms such as bacteria, viruses and fungi. It was therefore enormously beneficial to microbiologists when the field of nanotechnology began to emerge. In the 1980s, many significant technological developments were made, beginning with the scanning tunnelling microscope (STM) which enabled scientists to observe and manipulate individual atoms (the inventors of STM were later awarded a Nobel Prize). In the mid-1980s, this was followed by the development of atomic force microscopy (AFM), in which a very sharp probe (tip) touches the sample surface with ultra-small forces while raster scanning to obtain topographic images with a resolution of only a few nanometres. AFM is also an ultrasensitive force measuring technique, an approach known as AFM force spectroscopy. Here, force-distance curves are obtained by bringing the probe against the sample and then retracting it while monitoring the force. Attaching specific bioligands to the probe makes it possible to measure the localisation, strength and dynamics of single receptor-ligand interactions, such as staphylococcal adhesin-host protein bonds.
HAVE THERE BEEN ANY EUREKA MOMENTS IN YVES’ RESEARCH?
Of course! In fact, there are probably too many to mention here, but there was the discovery of the ultra-stable adhesion-host protein interactions; the finding of anti-adhesion molecules which could one day help complement antibiotics to treat multi-resistant superbugs; and the first evidence of a catch-bond between an adhesin on a living staphylococcal cell and its target protein. But the findings do not stop there! “For instance, we have done some very exciting work on yeasts, where we have unravelled the force-interaction that plays a role in mating: we now understand yeast sexuality better!” says Yves. “AFM technology is continuously improving, but recent advances in microbiology research include the improvement of imaging speeds, the possibility to do multiparametric imaging (images of topography, adhesion and stiffness generated simultaneously on live cells) and a new modality called fluidic force microscopy where microchanneled probes with micro- or nanosized apertures allow for fast manipulation and analysis of single cells.”
Clearly, this is a field ripe for innovation and new discoveries, so if you are interested in making several significant contributions to a particular branch of molecular and cellular biology, this could be the one for you!
WHAT DOES YVES FIND MOST REWARDING ABOUT HIS WORK?
Yves’ work is truly multidisciplinary, relying strongly on collaborations with microbiologists around the world, which provide clinical strains and bacterial mutants altered in specific adhesins, but also complement AFM data with results from traditional bioassays. It is rewarding to know his team’s work will likely have positive scientific, societal and economic impacts. On a more personal level, Yves is able to work closely with early career researchers who are highly motivated, and to help them achieve their ambitions and develop their skills as scientists. In particular, his former PhD student, David Alsteens, is now a professor at UCLouvain. They share the same instruments and belong to the same nanobio group, but David’s research, also funded by the ERC, mainly focuses on viruses.
WHAT ARE THE ISSUES FACING THE NEXT GENERATION OF NANOMICROBIOLOGISTS?
For those of you interested in pursuing a career in the field, you can expect to be working on problems such as how best to combine AFM with optical nanoscopy. “Optical nanoscopy is unique in that it can probe the localisation and motion of single molecules in live cells with a resolution in the range of a few tens of nanometres,” says Yves. “Combining AFM and optical nanoscopy will allow researchers to probe intracellular and extracellular structures with unprecedented resolutions, thus enabling researchers to study their organisation, dynamics and interactions in individual cells, at the single-molecule level and from the inside out.”
HOW TO BECOME INVOLVED IN NANOBIOPHYSICS AND MICROBIOLOGY
• UnderstandingNano includes an array of useful information relating to the wide variety of specific areas of interest within nanotechnology:
https://www.understandingnano.com/resources.html
• Societies are a good place to start researching a field from a broad perspective.
• The American Society for Microbiology (ASM) is the oldest and largest single life science membership organisation in the world: https://asm.org
• The Microbiology Society provides lots of useful information, including a careers page: https://microbiologysociety.org
PATHWAY FROM SCHOOL TO NANOTECH, BIOPHYSICS AND MICROBIOLOGY
Yves concedes that his field is so multidisciplinary that it is virtually impossible to suggest the correct pathway to take! However, studying biology, physics and chemistry courses will be of obvious use. At university, taking a bioengineering degree could be a good way to enter the nanobioworld.
Relevant degree subjects include physics, chemistry, biophysics, microbiology and engineering.
https://targetjobs.co.uk/careers-advice/job-descriptions/454437-nanoscientist-job-description
HOW DID YVES BECOME A NANOBIOPHYSICIST?
WHAT WERE YOUR INTEREST AS A CHILD?
I was always fascinated with nature – even from a young age – and I was often dreaming and living inside my head and its thoughts. As a teenager, I found I did not really align very well with my peers in that we appeared to have very different interests. But then, at the age of 16, I went into extreme caving, meaning you spend one week in an alpine cave. We made so many exciting discoveries of new rooms and passages. It really was a brilliant experience where I made many friends excited by caving, climbing and hiking.
DID YOU HAVE A FAVOURITE BOOK AS A CHILD?
It has always been music that has been my most passionate interest outside of science. My current music interests are wide and varied, from Pink Floyd to AC/DC, through to electronic and Baroque music!
WHO OR WHAT INSPIRED YOU TO BECOME A SCIENTIST?
When I was 10, I received my first microscope (which you can see on the first page of my website). It was a life-changing experience in many ways and I came to love looking at bacteria, etc. Later, as a student, PhD and postdoc fellow, I came to be massively inspired by my mentors which led me studying many different things – but the microscope was certainly a key moment in creating that initial motivation to find out more about the world. For as long as I can remember, I have thought of science as being an amazing way to express your imagination and creativity. One of my favourite quotes is from Albert Einstein: “Knowledge is limited. Imagination encircles the world.”
WHAT ATTRIBUTES HAVE MADE YOU A SUCCESSFUL SCIENTIST?
I approach all of my studies with passion, excitement, intensity and enthusiasm, which has served me well throughout my career. Then there is the fact I am hardworking – sometimes seven days a week – and we clearly do not do that for money! It is important to have an innate love for science and enjoy what you do.
HOW DO YOU OVERCOME OBSTACLES IN YOUR WORK?
You have to be patient when researching and accept that you will fail sometimes. Indeed, failures can lead to some top discoveries! In my spare time, I like to go jogging and do yoga – it is important to find a way to switch off at times. In the summer, I very much enjoy to hike solo in the mountains, sometimes for weeks.
WHAT IS YOUR PROUDEST CAREER ACHIEVEMENT?
A few years ago, I was awarded a European Research Council advanced grant from the European Union. This enabled me and my team to boost our research on staphylococcal adhesion and anti-adhesion using the tools of nanotechnology. The grant has led to some exciting publications and I am extremely happy about the opportunities it has provided and will likely continue to provide in the future. I am also very grateful for the opportunities I had to work with so many highly motivated team members as well as collaborators worldwide. Finally, the AFM-based nanobiophysics community is relatively small, meaning that conferences and meetings in the field are always exciting from the intellectual and social standpoints.
YVES’ TOP TIPS
01 Approach your studies with imagination, passion, excitement and enthusiasm. There is sometimes a belief that science relies only on well-established knowledge and ultra-critical thinking but in my experience, it is a field that also enables artistic pursuits.
02 Harnessing and developing the ability to collaborate with your peers and communicate effectively with your colleagues will serve you well throughout your career. Work to improve communication for a wide range of different reasons. Good English writing and speaking skills are essential as this is the international language of scientists.
03 The work of a scientist can be difficult and sometimes frustrating, but you should always try to have fun in everything you do!
Do you have a question for Yves?
Write it in the comments box below and Yves will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)