What if we understood the genetic causes of cancer?
Cancer is a disease caused by mutations in the genes within our cells. Professor Ian Prior, at the University of Liverpool in the UK, is trying to find out why some gene mutations are more likely to contribute to cancer than others. This knowledge could lead to the development of new treatments for this widespread and deadly disease
TALK LIKE A MOLECULAR ONCOLOGIST
ONCOLOGY – a branch of medicine relating to the study, prevention, diagnosis and treatment of cancer
ONCOGENIC – able to cause cancer
DNA – the molecule that stores the genetic information in our cells
RAS GENES – a group of genes involved in controlling cell growth and reproduction. The main members of the Ras gene family are KRAS, HRAS and NRAS. Many human cancers, such as colorectal, lung and pancreatic cancers, are driven by mutations in Ras genes
CRISPR – a gene editing technique used to introduce mutations into DNA
PROTEOMICS – the study of the structure, function and interactions of proteins produced by genes of a particular cell, tissue or organism
GENOMICS – the study of an organism’s genes (the genome), including interactions of genes with each other and
Ian Prior is a professor of molecular oncology and Head of the Liverpool Shared Research Facilities at the University of Liverpool. He has spent the past 25 years researching the causes of cancer and how this knowledge might be used to find new methods for treatment. His current research focuses on why a certain type of gene, called Ras, seems to contribute to a large percentage of cancer cases, and how defects in this gene could be corrected in order to slow or reverse the onset of disease.
WHAT CAUSES CANCER?
The DNA stored within cells carries the instructions for them to function, grow and reproduce. Cells reproduce by dividing into two new (daughter) cells. During division, the cell copies its DNA and passes it on to the daughter cells. “DNA is constantly being damaged by stresses that cells experience,” explains Ian. This damage causes mistakes to be made when the DNA is copied, creating mutations. For example, tobacco smoke contains chemicals that attach themselves to DNA, causing a mutation in the DNA when it is copied.
Usually, these mutations are corrected by the cell’s repair mechanisms, but sometimes errors slip through. Most of these mutations have no effect on the cell’s function, but mutations in genes that control cell division and growth can cause cancer. If a mutation causes these genes to become too active, it leads to uncontrolled cell growth and, therefore, a tumour will form.
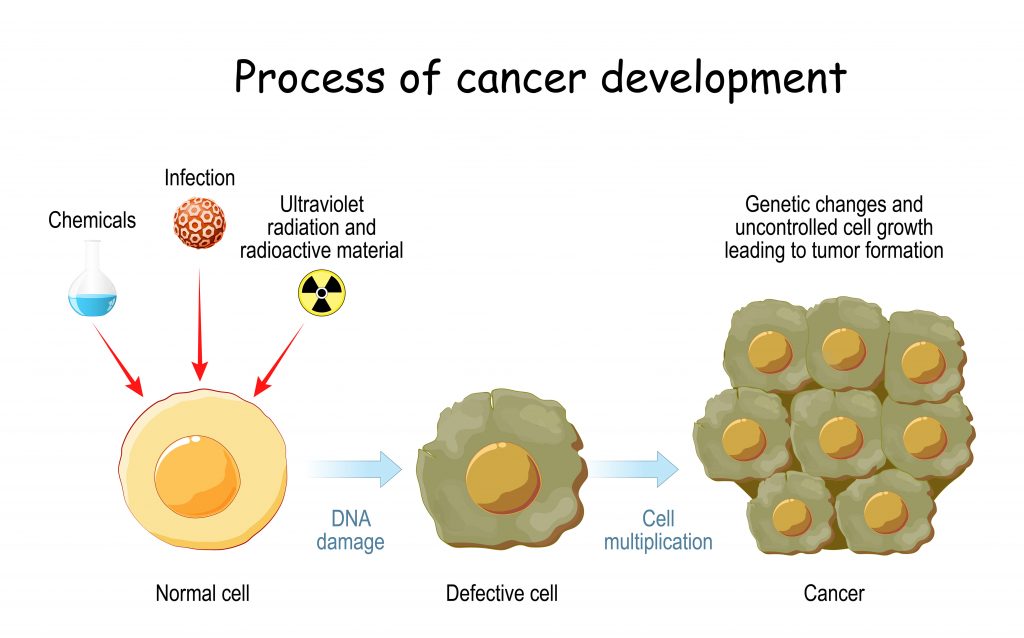
Sourced from Adobe, credit: designua
HOW ARE RAS GENES LINKED TO CANCER?
Ras genes control the activation of many other genes involved in cell division, growth and survival. Mutations in Ras genes can cause them to be permanently switched on, which means that they are constantly instructing the cell to divide and grow. Ras gene mutations are found in around 20% of all cancer patients, but are especially common in pancreatic (90%), colon (45%) and lung cancer (30%). There are three Ras genes: HRAS, KRAS and NRAS. KRAS is the most important for human development and is also the most frequently mutated. KRAS is the main focus of Ian’s research.
WHAT METHODS DOES IAN USE TO STUDY RAS GENES?
Ian’s team uses cell models and mice in its studies. The researchers use a gene editing technique called CRISPR to introduce various combinations of Ras mutations in mice, which, in turn, enables the researchers to find the mutations that are responsible for causing cancer. In traditional mouse-based studies, different mouse bloodlines would be created, each with a different mutation. Using CRISPR, Ian’s team can introduce multiple mutations into one mouse. “It means we can significantly reduce the number of mice that we use whilst at the same time increasing our ability to directly compare panels of cancer gene mutations,” says Ian.
The researchers also use genomics to study genes that are activated or deactivated by different mutations, and proteomics to study proteins regulated by Ras genes and how they are affected by different Ras mutations.
WHY IS CANCER SO DIFFICULT TO TREAT?
Reference
https://doi.org/10.33424/FUTURUM197
TALK LIKE A MOLECULAR ONCOLOGIST
ONCOLOGY – a branch of medicine relating to the study, prevention, diagnosis and treatment of cancer
ONCOGENIC – able to cause cancer
DNA – the molecule that stores the genetic information in our cells
RAS GENES – a group of genes involved in controlling cell growth and reproduction. The main members of the Ras gene family are KRAS, HRAS and NRAS. Many human cancers, such as colorectal, lung and pancreatic cancers, are driven by mutations in Ras genes
CRISPR – a gene editing technique used to introduce mutations into DNA
PROTEOMICS – the study of the structure, function and interactions of proteins produced by genes of a particular cell, tissue or organism
GENOMICS – the study of an organism’s genes (the genome), including interactions of genes with each other and
Ian Prior is a professor of molecular oncology and Head of the Liverpool Shared Research Facilities at the University of Liverpool. He has spent the past 25 years researching the causes of cancer and how this knowledge might be used to find new methods for treatment. His current research focuses on why a certain type of gene, called Ras, seems to contribute to a large percentage of cancer cases, and how defects in this gene could be corrected in order to slow or reverse the onset of disease.
WHAT CAUSES CANCER?
The DNA stored within cells carries the instructions for them to function, grow and reproduce. Cells reproduce by dividing into two new (daughter) cells. During division, the cell copies its DNA and passes it on to the daughter cells. “DNA is constantly being damaged by stresses that cells experience,” explains Ian. This damage causes mistakes to be made when the DNA is copied, creating mutations. For example, tobacco smoke contains chemicals that attach themselves to DNA, causing a mutation in the DNA when it is copied.
Usually, these mutations are corrected by the cell’s repair mechanisms, but sometimes errors slip through. Most of these mutations have no effect on the cell’s function, but mutations in genes that control cell division and growth can cause cancer. If a mutation causes these genes to become too active, it leads to uncontrolled cell growth and, therefore, a tumour will form.

Sourced from Adobe, credit: designua
HOW ARE RAS GENES LINKED TO CANCER?
Ras genes control the activation of many other genes involved in cell division, growth and survival. Mutations in Ras genes can cause them to be permanently switched on, which means that they are constantly instructing the cell to divide and grow. Ras gene mutations are found in around 20% of all cancer patients, but are especially common in pancreatic (90%), colon (45%) and lung cancer (30%). There are three Ras genes: HRAS, KRAS and NRAS. KRAS is the most important for human development and is also the most frequently mutated. KRAS is the main focus of Ian’s research.
WHAT METHODS DOES IAN USE TO STUDY RAS GENES?
Ian’s team uses cell models and mice in its studies. The researchers use a gene editing technique called CRISPR to introduce various combinations of Ras mutations in mice, which, in turn, enables the researchers to find the mutations that are responsible for causing cancer. In traditional mouse-based studies, different mouse bloodlines would be created, each with a different mutation. Using CRISPR, Ian’s team can introduce multiple mutations into one mouse. “It means we can significantly reduce the number of mice that we use whilst at the same time increasing our ability to directly compare panels of cancer gene mutations,” says Ian.
The researchers also use genomics to study genes that are activated or deactivated by different mutations, and proteomics to study proteins regulated by Ras genes and how they are affected by different Ras mutations.
WHY IS CANCER SO DIFFICULT TO TREAT?
Ras genes are not kinases, however, and the drugs that have been used to target kinases will not affect Ras-controlled processes. Ian explains: “Most successful drugs have been designed to bind to a pocket in a protein (like a key in a lock). To inhibit Ras, it needs to be prevented from interacting with partner proteins i.e., a drug needs to bind to the surface of Ras in a specific location. Instead of trying to find a key for a well understood lock, chemists have had to try to create drugs that can selectively bind to the smooth surface of Ras – it’s a very tough challenge.”
Recently, drugs that target a mutation (known as G12C) in KRAS, which is commonly found in lung cancer, have been developed and are currently being tested in clinical trials. Although these drugs are showing signs of being successful, one of Ian’s concerns is that cancers will become resistant to these new drugs. His team is testing different KRAS G12C inhibitors in lung cancer cells and investigating the mechanisms of resistance associated with each drug. The researchers have discovered that cells treated with these new inhibitors become resistant to some existing cancer drugs, but they have also identified combinations of drugs that could slow the onset of resistance.
HOW WILL IAN’S WORK LEAD TO IMPROVEMENTS FOR CANCER PATIENTS AND BENEFIT THE WIDER RESEARCH COMMUNITY?
“Ras controls a network of cancer-relevant genes. Understanding how the network operates and responds to therapies is important for treating cancer,” says Ian. “I want to understand what makes KRAS so oncogenic and why different Ras mutants are not all equally bad. Improved understanding will help therapeutic decision making and hopefully lead to the development of better treatments.”
Part of Ian’s role has been to establish a new centre known as the Liverpool Shared Research Facilities, which houses a wide range of imaging facilities and proteomics and genomics labs. Solving major scientific questions is no longer a solo endeavour so having all these facilities in one place has made it much easier for researchers with a diverse range of expertise to collaborate. Through these collaborative efforts involving a range of new technologies, cancer diagnosis and treatment will go from strength to strength.
 PROF IAN PRIOR
PROF IAN PRIOR
Professor of Molecular Oncology
Head of the Liverpool Shared Research Facilities
University of Liverpool, UK
FIELD OF RESEARCH: Molecular Oncology
RESEARCH PROJECT: Investigating Ras genes’ link to cancer and how defects in this family of genes could be corrected to slow or reverse the onset of disease
FUNDERS: Biotechnology and Biological Sciences Research Council, North West Cancer Research
 PROF IAN PRIOR
PROF IAN PRIOR
Professor of Molecular Oncology
Head of the Liverpool Shared Research Facilities
University of Liverpool, UK
FIELD OF RESEARCH: Molecular Oncology
RESEARCH PROJECT: Investigating Ras genes’ link to cancer and how defects in this family of genes could be corrected to slow or reverse the onset of disease
FUNDERS: Biotechnology and Biological Sciences Research Council, North West Cancer Research
ABOUT MOLECULAR ONCOLOGY
Molecular oncology is the study of cancer causes and treatments at the molecular scale. The aim is to identify the genes responsible for causing cancer and the mechanisms through which gene defects are translated into disease. This involves studying the chemical pathways in the cell that translate the genetic information of genes into cell processes, and why these go wrong in cancer cells. By understanding what goes wrong, we can develop new targets for cancer drugs or other treatments. Molecular oncology is a field which has advanced rapidly in recent years, as Ian explains:
Our understanding of cancer genetics via large-scale sequencing of cancer samples (exemplified by The Cancer Genome Atlas Program) has turbocharged our ability to identify the causes of cancer and how it evolves over time, new therapeutic targets and mechanisms of resistance to therapies. New clinical approaches are starting to include regular sequencing to get real time data on tumour responses to therapy. These provide the foundations for a future where each patient can be offered personalised therapies that are continuously optimised to keep the disease at bay.
I would definitely recommend a career in molecular oncology. I’d love to be at the start of my career again! It’s a very exciting time to be working in biomedical research and especially cancer research. There have already been significant advances in cancer treatment and, as a result, more than 50% of people diagnosed with cancer now survive. We are entering a new era where personalised medicine will become increasingly common and there are many opportunities in industry and academia to contribute to developing these treatments.
I have enormous respect for all of the people who have contributed to our understanding of cancer biology. It has been a huge team effort. A good book to read to find out about many of these achievements is The Emperor of All Maladies by Siddhartha Mukherjee. I am also grateful for the funding that has been invested to make this possible. My own research, in part, relies on funding from a local cancer charity (North West Cancer Research) and it’s always humbling when I meet the fundraisers who work so hard to make this possible.
EXPLORE A CAREER IN MOLECULAR ONCOLOGY
• The Institute of Cancer Research runs an internship scheme for undergraduate students every summer: www.icr.ac.uk/studying-and-training/undergraduate-vacation-scholarship-scheme
• “Don’t be afraid to contact people to ask for advice or an opportunity,” says Ian. Contact universities and laboratories near you to find out whether they offer tours, talks or internship opportunities.
• The American Society for Biochemistry and Molecular Biology has a useful resource for students that provides information on careers in these fields: www.asbmb.org/getmedia/f7c551d5-682e-44b1-a7a3-0cb3bcef9eac/asbmb-career-brochure-2017.pdf
• The average salary for an oncologist in the UK is £39,000 according to PayScale.com but this can be considerably higher for experienced researchers.
PATHWAY FROM SCHOOL TO MOLECULAR ONCOLOGIST
• A strong background in science subjects is important, in particular chemistry and biology.
• Many medical research projects involve analysing large datasets using computational techniques, so having some programming and statistics knowledge is useful. Most undergraduate science degrees now offer some form of training in these skills.
• Career Explorer offers an overview of oncology and cancer biology degree options: www.careerexplorer.com/degrees/oncology-and-cancer-biology-degree/
HOW DID IAN BECOME A MOLECULAR ONCOLOGIST?
WHAT DID YOU WANT TO BE WHEN YOU WERE YOUNGER?
I liked most subjects at school, but the sciences were the most interesting. I didn’t have a clear idea of the range of careers that you could have in those areas; it always seemed like a good idea to keep focusing on the things that I enjoyed the most.
YOU HAVE A BSC IN ZOOLOGY, AN MSC IN TOXICOLOGY AND A PHD IN MOLECULAR CELL BIOLOGY. WHY DID YOU TRANSITION FROM ZOOLOGY TO MOLECULAR CELL BIOLOGY?
Zoology was a very enjoyable degree, but it became obvious to me that there were many more lab-based jobs available if I wanted to have a career in scientific research. I had no fixed idea of the type of lab science I wanted to do, but that flexibility was an advantage when making the transition.
THINKING ABOUT STUDENTS WHO MAY NOT HAVE A CLEAR IDEA OF WHAT THEY WOULD LIKE TO DO CAREER-WISE, HOW EASY IS IT TO SWITCH BETWEEN DISCIPLINES?
Biomedical research requires a wide range of expertise to deliver big results and it’s not unusual to find people with chemistry, physical science, mathematics and computing backgrounds. Switching disciplines often involves retraining (e.g. via a postgraduate degree). Alternatively, take your specialised experience to a new discipline that needs it – such as programmers who help with big data analysis of cancer genetics patterns.
OF ALL YOUR PROFESSIONAL ACHIEVEMENTS, WHAT HAS BEEN YOUR PROUDEST MOMENT SO FAR?
I really value the opportunities that I have had to help my students and lab members to develop as scientists and then go on to achieve their career goals.
WHAT DO YOU LIKE TO DO IN YOUR SPARE TIME?
I like to draw. Research can be frustrating at times, not every idea works out and sometimes you work really hard and have nothing to show for it. Drawing always results in an end product and it’s a nice way to switch off.
IAN’S TOP TIPS
01 Be flexible. There are many routes to a career in science, but it is much harder if you are only prepared to consider one route.
02 Qualifications are important but also look for work experience, e.g. summer projects during your undergraduate degree. This will help you to decide if you like working in a lab.
03 Be open, share your ideas and work with the best people you can find. Science is a team activity.
Do you have a question for Ian?
Write it in the comments box below and Ian will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)