How can scientists clean up hydrogen production to help tackle climate change?
Hydrogen could be an important clean energy source in future, reducing greenhouse gas emissions and helping to combat climate change. However, currently, producing hydrogen fuel is not a clean process – most of the time, it generates greenhouse gases. Dr Nicolas Boscher and his team at the Luxembourg Institute of Science and Technology are using chemical engineering techniques – and taking inspiration from photosynthesis occurring in plants – to develop new polymers with the ability to produce hydrogen in a clean way
TALK LIKE A MATERIALS CHEMIST
GREENHOUSE GASES – gases such as carbon dioxide in the Earth’s atmosphere which trap heat, causing climate change
FOSSIL FUELS – fuels like coal, oil and gas that form from fossilised remains of plants and animals over millions of years and emit greenhouse gases when burned
CLEAN ENERGY – energy that does not generate pollutants or greenhouse gases
PHOTOCATALYST – a material which uses light energy to generate or accelerate a chemical reaction
CHLOROPHYLL – a green molecule found in plants that absorbs sunlight, powering photosynthesis
MICROPOROUS – a material containing tiny holes known as pores
POLYMER – a large molecule composed of many repeated subunits
SYNTHESIS – combining or joining many small parts to make a connected whole
Burning hydrogen does not produce greenhouse gases, which means that it has the potential to be a clean fuel powering cars, planes and other processes currently dependent on fossil fuels. However, the production of hydrogen fuels usually generates greenhouse gas emissions. Around 95% of hydrogen is currently produced from fossil fuels, limiting its potential to be a truly clean energy source.
Dr Nicolas Boscher and his international team at the Luxembourg Institute of Science and Technology combine their expertise from a range of scientific fields, aiming to find new, clean ways of producing hydrogen. Perfecting methods like these and cleaning up hydrogen production may help the world to reduce greenhouse gas emissions and tackle climate change.
A CLEAN FUEL
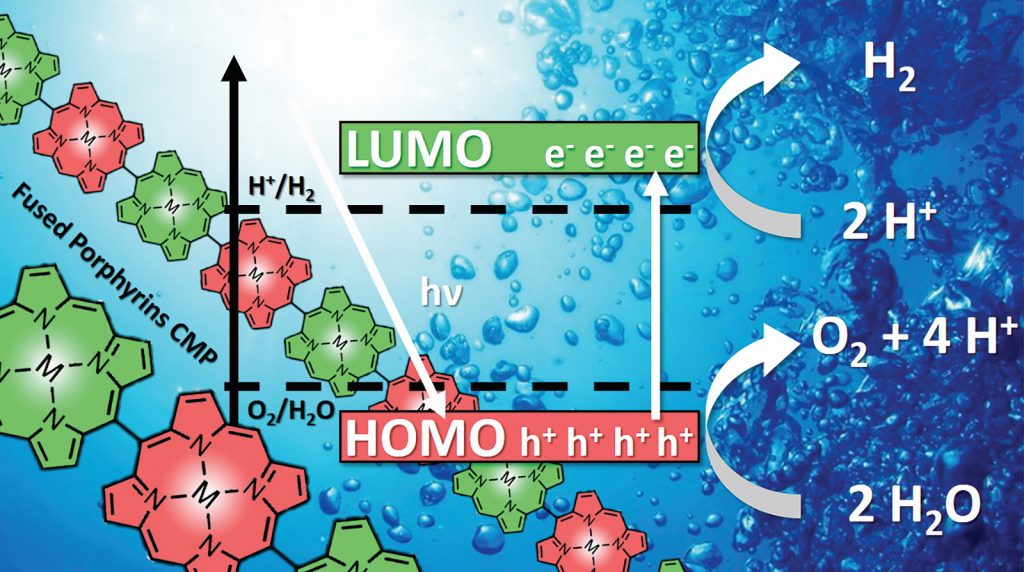
Most hydrogen is currently produced from methane or natural gas, in a process that generates carbon dioxide – a greenhouse gas. Scientists are trying to develop alternative hydrogen production methods that do not generate greenhouse gas emissions so that hydrogen can be used as a clean fuel. For Nicolas and his team, this involves working on a process called photocatalytic water splitting. Their methods mimic part of the process of photosynthesis whereby plants use energy from the sun to transform water (H2O) and carbon dioxide (CO2) into oxygen (O2) and sugar (C6H12O2). Nicolas explains, “For over three billion years nature has been implementing water splitting using sunlight”. Photocatalytic water splitting is artificial photosynthesis that uses light to split H2O molecules into H2 and O2.
MIMICKING PHOTOSYNTHESIS
Nicolas explains that he and his team create new polymer photocatalysts by joining together smaller chlorophyll-like molecules or other strongly coloured molecules. The resulting photocatalyst must have two key properties: 1) it can absorb light and turn it into energy, 2) it is microporous with active sites that molecules can bind to so that chemical reactions can take place. Nicolas says, “Imagine an intensely coloured polymer foil on which hydrogen and oxygen bubbles form when immersed in water, under sunlight.”
SYNTHESISING NEW POLYMERS
“The synthesis of our photocatalytic polymers is not straightforward,” says Nicolas. Some of the molecules used have poor solubility – they do not dissolve well in liquids – which means that it is difficult to synthesise usable polymer photocatalysts using a traditional ‘wet chemistry’ approach (with mainly liquid chemicals).
To overcome this problem, Nicolas and his team use molecules in a gas state to synthesise their polymers while modifying them to be useful. Nicolas says this gas phase approach opens the doors to other opportunities, like trying different molecules as polymer subunits and engineering polymers in new ways. Nicolas has been working on gas phase synthesis of new materials since he started his PhD in 2004, collaborating with different scientists in the UK, USA and Luxembourg to develop methods and find new solutions to research problems. This formed the foundations for the work of his current research team, which combines a range of expertise to develop methods for producing photocatalytic polymers. THE
BENEFITS
If hydrogen can be produced in a way that does not produce greenhouse gases, it could be an entirely clean fuel used in a wide range of applications, contributing to the fight against climate change. For example, while transitioning from petrol and diesel to electric cars could be another important way of reducing greenhouse gas emissions, large vehicles like buses, trains, trucks, ships and planes would require prohibitively heavy batteries (~1500 kg) to be powered by electricity. Hydrogen fuel could be a much more useful alternative. Hydrogen can also be used for clean heat and power generation.
Aside from hydrogen production, the research being done by Nicolas’ team could also provide the foundations for a wide range of new approaches in chemistry. Photocatalysts could be engineered to mimic other biological processes and power chemical reactions, such as producing plastics from carbon dioxide. Nicolas says that this would represent a giant step towards much more sustainable approaches in chemistry which do not use fossil fuels, known as ‘green chemistry’, shaping the work of future chemists.
 DR NICOLAS BOSCHER
DR NICOLAS BOSCHER
Lead Researcher at the Luxembourg Institute of Science and Technology, Luxembourg
FIELD OF RESEARCH: Materials Chemistry
RESEARCH PROJECT: CLEANH2, TODAM & POLYPORPH – developing materials chemistry techniques for the clean production of hydrogen fuels, reducing greenhouse gas emissions
FUNDERS: European Commission, Luxembourg National Research Fund (FNR)
The CLEANH2 project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 865985). The TODAM project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 101031568. The POLYPORPH project has received funding from the Luxembourg National Research Fund (FNR) under the CORE programme (C15/MS/10340560).
NICOLAS’ TOP TIPS
01 Be determined and methodical. Developing a rational and structured approach will provide you with the courage to go to unexplored fields and tackle challenges.
02 Complement your skills. Do not stick with what you already know or what you have done in the past. Learn from your colleagues, develop new skills and merge them with your expertise to explore things from a new perspective.
03 Keep an open mind and learn from your mistakes. Consider every opinion, especially criticisms, which are often excellent opportunities to improve yourself. Do not let failures and mistakes stop you. Analyse them, learn from them and adapt your approach to succeed next time.
MEET THE TEAM

DR DRIALYS CARDENAS-MORCOSO
NATIONALITY: CUBAN
AREAS OF RESEARCH: RADIOCHEMISTRY, APPLIED PHYSICS
I joined CLEANH2 to contribute to understanding the structure and properties of the molecules used in the project.
Since childhood, I have been curious and keen to understand nature’s processes. As I grew up, I participated in science projects at school. During my master’s and PhD, I focused on the synthesis and characterisation of materials for the development of efficient light-powered devices and particularly solar fuels production. Now, I am bringing what I have learned to CLEANH2, helping to understand and develop solar hydrogen production systems.
Growing up in Cuba, I was impressed by the scientific advances in my country, particularly in biotechnology and in the development of materials, despite limited resources. I believe that the investment in technical-scientific development in Cuba over recent decades motivated many people of my generation to lean towards scientific careers.
I am also very concerned about environmental issues and their consequences, especially for future generations. I decided, as far as possible, to gear my scientific career towards developing approaches that can contribute to a sustainable future.
I feel very proud of contributing to projects aiming to develop practical devices for producing clean fuels from abundant and feasible materials. My future goal is to lead a research project developing new materials and approaches than can help to meet, in a safe and environmentally friendly way, current energy demands.

DR MAREK KRZYSZTOF CHARYTON
NATIONALITY: POLISH
AREAS OF RESEARCH: ORGANIC CHEMISTRY (FUNCTIONAL DYES), MATERIAL SCIENCES
In the TODAM project, I am responsible for designing, synthesising and understanding basic properties of synthetic organic (carbon-based) dyes.
I was raised in a region known as ‘the Green Lungs of Poland’ because of its vast forest, post-glacial lakes, basins and river valleys. I used to spend a lot of time in woods learning about wildlife, different environments and how ecology relates to modern society.
As child, I often watched documentaries and read books related to botany and zoology. I found the most fascinating parts were related to discovering a new functionality of a known species. This led me towards nature-inspired organic chemistry and bio-based materials.
For me, the most rewarding thing in the field of synthesis of organic dyes is when non-coloured compounds (simple and small molecules) react to form products with intense colours (larger molecules with more complex structure). The challenge is that even small differences in the reaction conditions or traces of impurity in the substances used can lead to results that can’t be reproduced. However, sometimes an unfortunate mistake leads to a very fortunate discovery. A scientist should always try their best to understand what has happened in the reaction flask!

DR KAMAL BABA
NATIONALITY: ALGERIAN
AREAS OF RESEARCH: CHEMICAL AND PROCESS ENGINEERING
As a kid, I was always curious about how things work. I wanted to understand the workings of artificial things such as an electric toy car, a home radio, the dynamo of a bike, but also natural phenomena such as day and night, seasons, rain, and snow. I learned lots at school, but also learned by watching TV and through life experience – when something broke at home, I was always happy trying to fix it!
I came to in the field of science very late and by chance. When I decided to study chemical engineering, I was dreaming of being an engineer in the oil and gas industry, which is one of the best sectors to work in in Algeria. However, after finishing my first degree I decided to continue my studies and live abroad. During my master’s in France, I had several opportunities to visit the research lab where many of the lecturers were working. It was very interesting to see how scientists and their students, engineers and technicians work. At the end of my master’s, I was convinced by one of my lecturers to do a research internship in the lab with them. It was a very exciting and motivating experience, and it felt natural to continue the experience as a PhD researcher.

MAX HEIDERSCHEID, MSC
NATIONALITY: LUXEMBOURGISH
AREA OF RESEARCH: INORGANIC AND ORGANIC CHEMISTRY
Reference
https://doi.org/10.33424/FUTURUM198
TALK LIKE A MATERIALS CHEMIST
GREENHOUSE GASES – gases such as carbon dioxide in the Earth’s atmosphere which trap heat, causing climate change
FOSSIL FUELS – fuels like coal, oil and gas that form from fossilised remains of plants and animals over millions of years and emit greenhouse gases when burned
CLEAN ENERGY – energy that does not generate pollutants or greenhouse gases
PHOTOCATALYST – a material which uses light energy to generate or accelerate a chemical reaction
CHLOROPHYLL – a green molecule found in plants that absorbs sunlight, powering photosynthesis
MICROPOROUS – a material containing tiny holes known as pores
POLYMER – a large molecule composed of many repeated subunits
SYNTHESIS – combining or joining many small parts to make a connected whole
Burning hydrogen does not produce greenhouse gases, which means that it has the potential to be a clean fuel powering cars, planes and other processes currently dependent on fossil fuels. However, the production of hydrogen fuels usually generates greenhouse gas emissions. Around 95% of hydrogen is currently produced from fossil fuels, limiting its potential to be a truly clean energy source.
Dr Nicolas Boscher and his international team at the Luxembourg Institute of Science and Technology combine their expertise from a range of scientific fields, aiming to find new, clean ways of producing hydrogen. Perfecting methods like these and cleaning up hydrogen production may help the world to reduce greenhouse gas emissions and tackle climate change.
A CLEAN FUEL
Most hydrogen is currently produced from methane or natural gas, in a process that generates carbon dioxide – a greenhouse gas. Scientists are trying to develop alternative hydrogen production methods that do not generate greenhouse gas emissions so that hydrogen can be used as a clean fuel. For Nicolas and his team, this involves working on a process called photocatalytic water splitting. Their methods mimic part of the process of photosynthesis whereby plants use energy from the sun to transform water (H2O) and carbon dioxide (CO2) into oxygen (O2) and sugar (C6H12O2). Nicolas explains, “For over three billion years nature has been implementing water splitting using sunlight”. Photocatalytic water splitting is artificial photosynthesis that uses light to split H2O molecules into H2 and O2.
MIMICKING PHOTOSYNTHESIS
Nicolas explains that he and his team create new polymer photocatalysts by joining together smaller chlorophyll-like molecules or other strongly coloured molecules. The resulting photocatalyst must have two key properties: 1) it can absorb light and turn it into energy, 2) it is microporous with active sites that molecules can bind to so that chemical reactions can take place. Nicolas says, “Imagine an intensely coloured polymer foil on which hydrogen and oxygen bubbles form when immersed in water, under sunlight.”
SYNTHESISING NEW POLYMERS
“The synthesis of our photocatalytic polymers is not straightforward,” says Nicolas. Some of the molecules used have poor solubility – they do not dissolve well in liquids – which means that it is difficult to synthesise usable polymer photocatalysts using a traditional ‘wet chemistry’ approach (with mainly liquid chemicals).
To overcome this problem, Nicolas and his team use molecules in a gas state to synthesise their polymers while modifying them to be useful. Nicolas says this gas phase approach opens the doors to other opportunities, like trying different molecules as polymer subunits and engineering polymers in new ways. Nicolas has been working on gas phase synthesis of new materials since he started his PhD in 2004, collaborating with different scientists in the UK, USA and Luxembourg to develop methods and find new solutions to research problems. This formed the foundations for the work of his current research team, which combines a range of expertise to develop methods for producing photocatalytic polymers. THE
BENEFITS
If hydrogen can be produced in a way that does not produce greenhouse gases, it could be an entirely clean fuel used in a wide range of applications, contributing to the fight against climate change. For example, while transitioning from petrol and diesel to electric cars could be another important way of reducing greenhouse gas emissions, large vehicles like buses, trains, trucks, ships and planes would require prohibitively heavy batteries (~1500 kg) to be powered by electricity. Hydrogen fuel could be a much more useful alternative. Hydrogen can also be used for clean heat and power generation.
Aside from hydrogen production, the research being done by Nicolas’ team could also provide the foundations for a wide range of new approaches in chemistry. Photocatalysts could be engineered to mimic other biological processes and power chemical reactions, such as producing plastics from carbon dioxide. Nicolas says that this would represent a giant step towards much more sustainable approaches in chemistry which do not use fossil fuels, known as ‘green chemistry’, shaping the work of future chemists.
 DR NICOLAS BOSCHER
DR NICOLAS BOSCHER
Lead Researcher at the Luxembourg Institute of Science and Technology, Luxembourg
FIELD OF RESEARCH: Materials Chemistry
RESEARCH PROJECT: CLEANH2, TODAM & POLYPORPH – developing materials chemistry techniques for the clean production of hydrogen fuels, reducing greenhouse gas emissions
FUNDERS: European Commission, Luxembourg National Research Fund (FNR)
The CLEANH2 project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 865985). The TODAM project
has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 101031568. The POLYPORPH project has received funding from the Luxembourg National Research Fund (FNR) under the CORE programme (C15/MS/10340560).
NICOLAS’ TOP TIPS
01 Be determined and methodical. Developing a rational and structured approach will provide you with the courage to go to unexplored fields and tackle challenges.
02 Complement your skills. Do not stick with what you already know or what you have done in the past. Learn from your colleagues, develop new skills and merge them with your expertise to explore things from a new perspective.
03 Keep an open mind and learn from your mistakes. Consider every opinion, especially criticisms, which are often excellent opportunities to improve yourself. Do not let failures and mistakes stop you. Analyse them, learn from them and adapt your approach to succeed next time.

DR DRIALYS CARDENAS-MORCOSO
NATIONALITY: CUBAN
AREAS OF RESEARCH: RADIOCHEMISTRY, APPLIED PHYSICS
I joined CLEANH2 to contribute to understanding the structure and properties of the molecules used in the project.
Since childhood, I have been curious and keen to understand nature’s processes. As I grew up, I participated in science projects at school. During my master’s and PhD, I focused on the synthesis and characterisation of materials for the development of efficient light-powered devices and particularly solar fuels production. Now, I am bringing what I have learned to CLEANH2, helping to understand and develop solar hydrogen production systems.
Growing up in Cuba, I was impressed by the scientific advances in my country, particularly in biotechnology and in the development of materials, despite limited resources. I believe that the investment in technical-scientific development in Cuba over recent decades motivated many people of my generation to lean towards scientific careers.
I am also very concerned about environmental issues and their consequences, especially for future generations. I decided, as far as possible, to gear my scientific career towards developing approaches that can contribute to a sustainable future.
I feel very proud of contributing to projects aiming to develop practical devices for producing clean fuels from abundant and feasible materials. My future goal is to lead a research project developing new materials and approaches than can help to meet, in a safe and environmentally friendly way, current energy demands.

DR MAREK KRZYSZTOF CHARYTON
NATIONALITY: POLISH
AREAS OF RESEARCH: ORGANIC CHEMISTRY (FUNCTIONAL DYES), MATERIAL SCIENCES
In the TODAM project, I am responsible for designing, synthesising and understanding basic properties of synthetic organic (carbon-based) dyes.
I was raised in a region known as ‘the Green Lungs of Poland’ because of its vast forest, post-glacial lakes, basins and river valleys. I used to spend a lot of time in woods learning about wildlife, different environments and how ecology relates to modern society.
As child, I often watched documentaries and read books related to botany and zoology. I found the most fascinating parts were related to discovering a new functionality of a known species. This led me towards nature-inspired organic chemistry and bio-based materials.
For me, the most rewarding thing in the field of synthesis of organic dyes is when non-coloured compounds (simple and small molecules) react to form products with intense colours (larger molecules with more complex structure). The challenge is that even small differences in the reaction conditions or traces of impurity in the substances used can lead to results that can’t be reproduced. However, sometimes an unfortunate mistake leads to a very fortunate discovery. A scientist should always try their best to understand what has happened in the reaction flask!

DR KAMAL BABA
NATIONALITY: ALGERIAN
AREAS OF RESEARCH: CHEMICAL AND PROCESS ENGINEERING
As a kid, I was always curious about how things work. I wanted to understand the workings of artificial things such as an electric toy car, a home radio, the dynamo of a bike, but also natural phenomena such as day and night, seasons, rain, and snow. I learned lots at school, but also learned by watching TV and through life experience – when something broke at home, I was always happy trying to fix it!
I came to in the field of science very late and by chance. When I decided to study chemical engineering, I was dreaming of being an engineer in the oil and gas industry, which is one of the best sectors to work in in Algeria. However, after finishing my first degree I decided to continue my studies and live abroad. During my master’s in France, I had several opportunities to visit the research lab where many of the lecturers were working. It was very interesting to see how scientists and their students, engineers and technicians work. At the end of my master’s, I was convinced by one of my lecturers to do a research internship in the lab with them. It was a very exciting and motivating experience, and it felt natural to continue the experience as a PhD researcher.

MAX HEIDERSCHEID, MSC
NATIONALITY: LUXEMBOURGISH
AREA OF RESEARCH: INORGANIC AND ORGANIC CHEMISTRY
Growing up in the countryside in Luxembourg, I have always been interested in natural phenomena. High school awakened my interest in natural sciences, especially chemistry. I did my bachelor’s degree in inorganic chemistry where I took part in a project, synthesising catalysts that are able to produce hydrogen by artificial photosynthesis. I continued with my master’s in bioorganic chemistry with focus on synthesising modified building blocks for DNA and RNA. My PhD research combines all my fields of interest, focusing on the goal of clean fuels for future.
The most exhausting and rewarding thing about chemistry is that it consists of several fields and is very broad! It is quite challenging to build up basic knowledge at first, but when you can connect all the knowledge it allows you to understand many natural phenomena. A big advantage of natural sciences and especially chemistry and materials science is that you can apply what you have learned and obtain physical proof of the applied knowledge.
Although I am still at the very beginning of my scientific career, I am very proud of the fact that I have a bachelor’s degree in inorganic chemistry, a master’s degree in bio-organic chemistry and am now doing a doctorate in materials science. At each of these stages, I have ventured into new waters to pursue my current areas of interest. I am also very proud to be involved in a project that aims to produce renewable energy to help make the world a more sustainable place.
THE TEAM’S TOP TIPS
01 Be disciplined and tenacious – in scientific research, you sometimes have to keep working hard even when you don’t see immediate success. But if you persevere, the outcomes can be very rewarding.
02 Be curious, and don’t shy away from exploring challenging questions as they may be the most important ones to answer.
03 Be passionate! As Steve Jobs said, “The only way to do great work, is to love what you do”.
EXPLORE A CAREER IN MATERIALS CHEMISTRY
• Materials chemists use chemistry techniques to understand how materials work, and to design and synthesise interesting new materials which will be applied for useful purposes.
• It is common for materials chemists to work as part of a team with a mixture of expertise, such as engineers and physicists.
• The Luxembourg National Research Fund has lots of useful information on its site, including details of science events.
• Salaries can vary depending on where materials chemists work, for example in a university or another organisation. According to www.glassdoor.co.uk, the national average salary for a researcher is €61,000 per year in Luxembourg.
PATHWAY FROM SCHOOL TO MATERIALS CHEMISTRY
• The members of Nicolas’ team have varied backgrounds and have bachelor’s and postgraduate degrees in different fields related to chemistry, physics and chemical engineering. However, they have one important thing in common – they all pursued their passions and interests, which lead them to bring their individual skillsets to the team’s research activities.
• Being involved in a project like CLEANH2 requires an undergraduate degree (usually followed by a postgraduate degree) in a related field – so it is worth researching different types of chemistry and materials science courses to see what sparks your interest. As Max explains, “It is important to have a good general knowledge of chemistry, as well as physics and mathematics. By gaining this basic knowledge, you quickly realise what you are most interested in and which path and specialisations you will take.”
• Drialys highlights the importance of networking, meeting and talking to people when exploring a career in this field. She advises, “Don’t miss the chance to participate in science festivals or young scientist conferences usually held by the universities. In addition, you can look for opportunities of internships in laboratories.”
• More information on pursuing a career in chemistry can be found here: nationalcareers.service.gov.uk/job-profiles/chemist
Do you have a question for Nicolas and the team?
Write it in the comments box below and Nicolas and the team will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)