How do rare genetic disorders affect vitamin B12 metabolism and embryonic development?
Vitamin B12 plays a crucial role in our growth and development. Without it, our bodies cannot produce healthy blood cells or maintain a functioning nervous system. For most people, a balanced diet provides enough vitamin B12 to stay healthy, but for some, rare genetic disorders mean their bodies cannot use this vital vitamin properly. Dr Ross Poché from Baylor College of Medicine in Houston, Texas, USA, uses mouse models to study these disorders, understand how they disrupt embryonic brain development and explore new ways of treating them.
Talk like a rare disease researcher
Coenzyme — a molecule that helps an enzyme do its job effectively
DNA — the molecule that contains the genetic instructions for building and maintaining living organisms
Enzyme — a protein that speeds up chemical reactions in the body
Gene — a segment of DNA that provides instructions for making proteins
Genetic mutation — a change in the DNA sequence that can affect how proteins are made or function
Neural tube — the early embryonic structure that develops into the brain and spinal cord
Prenatal vitamins — nutritional supplements taken during pregnancy to support the healthy development of the baby
Vitamins are a group of essential nutrients that the body needs in order to function properly. Although our bodies can make some vitamins — for example, vitamin D is produced in the skin when exposed to sunlight — most of them must come from the food we eat.
Vitamin B12 is crucial for our health and is absorbed mainly from animal products, such as meat, eggs and dairy. It is essential for making new red blood cells and maintaining a healthy nervous system, so people following plant-based diets need fortified foods or supplements to stay healthy. Without vitamin B12, nerves can become permanently damaged, and, in extreme cases, death can occur.
During pregnancy, the need for B12 is even greater. “A developing embryo relies on a constant supply of B12 to build the brain, spinal cord and other organs,” says Dr Ross Poché at Baylor College of Medicine. “B12 deficiency during this time can result in serious birth defects and lifelong disabilities, which is why prenatal vitamins are so important.”
What happens when the body cannot use B12 properly?
“In rare cases, babies can suffer from severe developmental disorders caused by vitamin B12 deficiency, even if their B12 blood levels appear normal,” explains Ross. “These are genetic disorders where the body is unable to use B12 properly, despite having enough of it.”
Ross’s lab studies two such conditions. The first is called ‘combined methylmalonic acidaemia and homocystinuria type C’, or cblC for short. In this disorder, a baby’s cells are unable to convert vitamin B12 into its active form, causing the body to act as if it is severely lacking the vitamin. The second condition, known as cblX, is a related disorder that causes similar effects through a different cellular pathway. Both disorders disrupt brain development and overall health during early life.
How do these disorders affect embryonic development?
Genetic disorders like cblC and cblX, which affect how cells use vitamin B12, cause much more severe developmental problems than dietary B12 deficiency. Unlike a lack of B12 from food – which can often be treated with supplements – these genetic conditions prevent the body from properly using the vitamin, so supplements do not work. Babies with cblC or cblX often suffer from permanent brain and nervous system defects that significantly impact their quality of life.
Our complex brain develops from a simple embryonic structure called the neural tube, which must grow and form many different types of neurons and cells essential for the body’s functions. “This is a very complex process, and it is currently unclear precisely how vitamin B12 deficiencies impact it,” says Ross. “My lab hopes to shed some light on this question.”
What causes cblC and cblX, and how do they affect the body?
Genetic mutations are changes in our DNA sequence – the unique code in our genes that tells cells how to make proteins. Even small changes can affect how these proteins are produced or function. In cblC, a mutation in a gene called MMACHC disrupts the process that converts vitamin B12 into its active forms, which act as coenzymes in important cellular processes. Without these coenzymes, the cellular processes begin to break down.
“Imagine your brain is a city that must build and maintain huge neighbourhoods, and each neuron is a house in one of those neighbourhoods,” says Ross. “The workers in charge of building, fixing and cleaning these houses are enzymes. However, some of these enzymes can’t do their jobs alone — they need special power tools called coenzymes.” In cblC, vitamin B12 is not activated by MMACHC, meaning these coenzyme power tools do not work properly, and the enzymes are left unable to carry out essential tasks. This means the cells cannot build or repair DNA and proteins effectively, or clear away harmful waste. The rapidly growing neighbourhoods of the developing embryonic brain are especially vulnerable to this breakdown in cellular work.
“On the other hand, cblX is caused by mutations in another gene that acts like the city’s electrical grid manager,” explains Ross. “This gene ensures that enough MMACHC is produced to keep all the B12 power tools charged and ready.” If it is not working correctly, the city’s power stations fail, MMACHC levels drop, and the power tools sit idle – even if vitamin B12 is present in the body. The result is the same: stalled construction, toxic build-up, and increasing damage in the most delicate parts of the brain and nervous system.
How do mouse models help Ross understand these disorders?
Studying rare genetic diseases like cblC and cblX in humans can be very difficult because patient samples are limited and complex. That is why Ross and his team use mouse models – genetically modified mice that carry the same mutations found in human patients. While cell cultures (human cells grown in a dish) have led to advancements in research, they cannot replicate the complexity of a whole living body, where cells constantly interact with tissues, organs, hormones and the immune system.
Reference
https://doi.org/10.33424/FUTURUM637
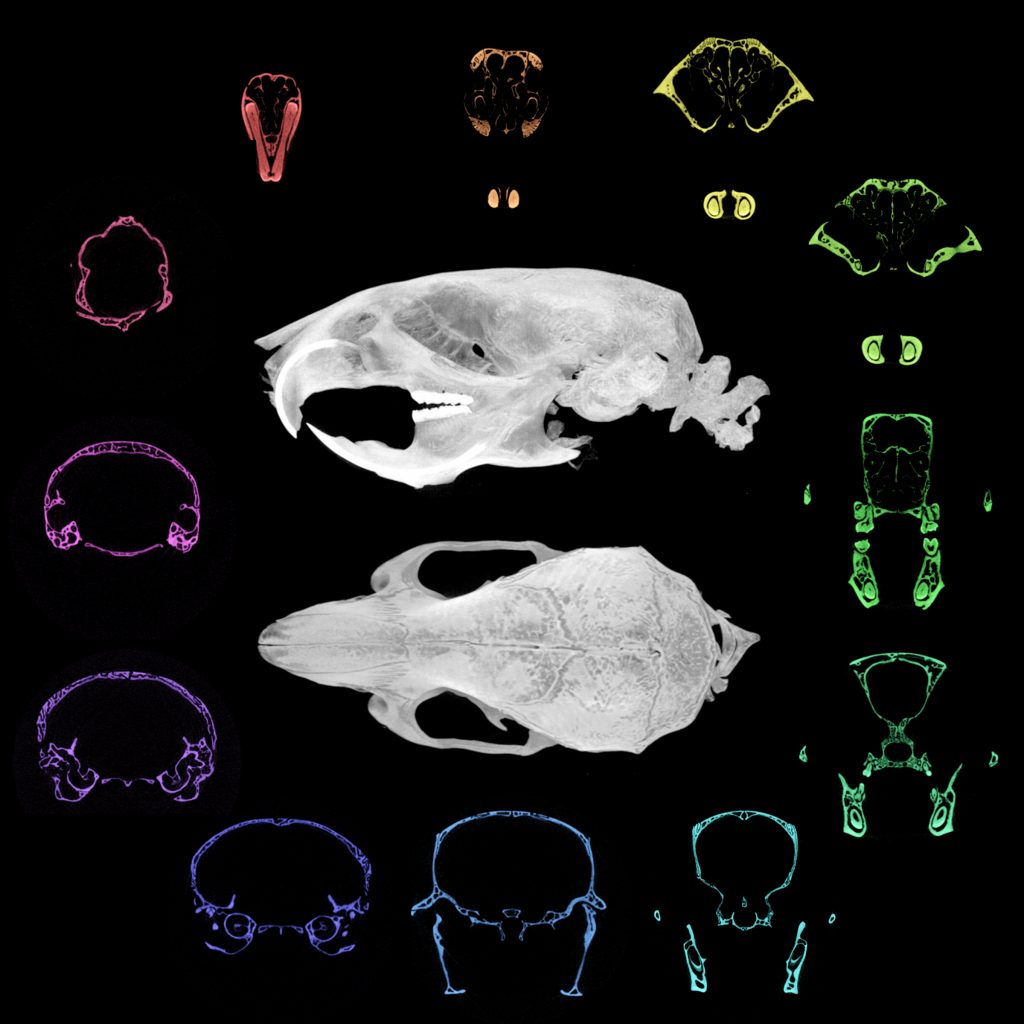
Micro-computed tomography of the adult mouse craniofacial skeleton, highlighting different bone structures (coloured). Credit: Dr Tiffany Chern (previous graduate student at the Poché lab)
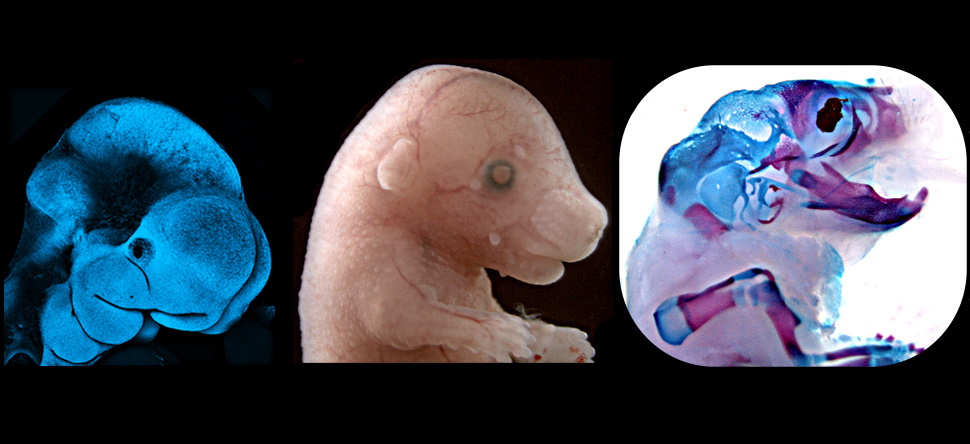
“Mice share a high degree of genetic similarity with humans, and they allow us to study the effects of specific mutations on the entire organism,” says Ross. “In my lab, we create genetically modified mice that carry the same mutations that cause rare disorders like cblC and cblX.”
Their recent studies on cblX mice revealed not only the expected nervous system problems but also new craniofacial defects – changes in the shape of the skull and face. This discovery broadens understanding of how the disorders impact different tissues and could guide the development of treatments that address both metabolic and structural problems.
“Without the proper nutrients or without the ability to use those nutrients properly, embryonic development can go off track in profound ways,” says Ross. “We’re still discovering all the different ways in which vitamins help our bodies grow and stay healthy. Studying rare diseases like cblC and cblX can teach us broader lessons about nutrition, genetics and early development.”
 Dr Ross Poché
Dr Ross Poché
Associate Professor of Integrative Physiology, Baylor College of Medicine, Houston, Texas, USA
Fields of research: Rare disease research, mouse genetics, developmental biology
Research project: Using mouse models to investigate rare genetic diseases that cause vitamin B12 deficiency
Funders: US National Institutes of Health (NIH); National Institute of Child Health and Human Development (NICHD, grant R01 HD116761); National Eye Institute (NEI, grant R21 EY035812)
About rare disease research
Rare disease research focuses on understanding uncommon genetic conditions that often arise from mutations in a single gene. Because these diseases are complex and affect multiple body systems, scientists rely on advanced tools to study them. Mouse models have become essential in this field because mice share many genetic and biological similarities with humans. By introducing the same mutations found in human patients into mice, researchers can observe how these changes impact the whole organism throughout development and into adulthood. “While alternative methods like cell cultures are valuable tools in research, they don’t replace mouse models — they complement them,” says Ross. “For now, studying rare diseases in the context of a living system is still the most powerful and reliable way to move from basic science to real therapies that improve lives.”
Ethical considerations are central to animal research. Scientists follow strict guidelines to ensure that studies involving mice are carried out responsibly and humanely. Before any research begins, proposals undergo careful review to confirm that animal use is necessary and justified. Researchers also adhere to the principles of replacement (using alternatives when possible), reduction (minimising the number of animals used), and refinement (improving methods to reduce pain and distress). “We never take animal research lightly,” says Ross. “Our goal is not only to advance science but to do so responsibly and respectfully, acknowledging that animals have made many of the greatest medical breakthroughs possible.”
“Studying rare diseases is essential not only because it brings hope to patients and families who have been historically overlooked, but also because these conditions often serve as powerful models for understanding fundamental biology,” says Ross. “Many rare diseases are caused by mutations in a single gene, making them ideal for uncovering how specific genes and pathways function in health and disease.” Insights gained from rare disease research often highlight biological pathways relevant to more common illnesses such as cancer, heart disease and neurodegeneration. Additionally, innovative technologies, such as gene editing and precision medicine, frequently originate in this field, benefiting a broad spectrum of medical research.
Pathway from school to rare disease research
“Fundamental courses in biology, chemistry, physics and statistics are important, but don’t overlook the humanities — areas like literature, history, philosophy, visual art and the performing arts,” says Ross. “The best scientists aren’t just skilled at doing science — they’re also excellent communicators, creative thinkers and problem-solvers.”
“As soon as possible, reach out to professors doing research in the field for intern positions in their labs,” says Ross. “Many of us are happy to mentor excited and engaged budding biomedical researchers.”
Explore careers in rare disease research
Professions in rare disease research include academic scientists, physicians and genetic counsellors, who help families understand inherited diseases, explain testing options, and provide emotional support during important medical decisions.
If you are interested in rare diseases, some useful organisations to explore include the National Institutes of Health Office of Rare Diseases Research, the National Organization for Rare Disorders, the Undiagnosed Diseases Network and the European Organization for Rare Diseases.
Meet Ross
Growing up as an identical triplet, I spent a lot of time being grouped together with my brothers — people often saw us as one unit instead of three individuals. But, by high school, our personalities started to shine through more clearly, and we worked hard to show we were different. Around that same time, I read about Dolly the sheep, the first mammal cloned from an adult cell. She was described as a genetic copy of the sheep she came from. That got me thinking: if Dolly was a clone, and I’m an ‘identical’ triplet, does that mean I’m a clone of my brothers? That moment was eye-opening. I realised that even with nearly identical DNA, my brothers and I were clearly not the same — and that fascinated me.
As a teenager, I had many interests, including biology — but I was especially drawn to visual art and the idea of visually capturing aspects of nature and the human experience in ways no one had seen before. That same creative impulse carries over into science. Just as artists use paintbrushes to reveal the unseen, scientists use technology and experimentation to uncover the hidden patterns of the natural world. This blend of curiosity and visual thinking ultimately led me to become a developmental biologist.
One of the best parts of this work is the intellectual freedom — the ability to ask big questions and pursue research that genuinely interests me. I also really enjoy the culture of the lab. It doesn’t feel like a traditional job; it feels like I’m just living my life, being myself, while also contributing something meaningful to the world.
In college, I worked in a lab focused on mapping mutations that cause Usher Syndrome Type 1 (USH1) — a rare congenital disorder that disproportionately affects infants in the Cajun population of Louisiana, causing profound hearing and vision loss. I often interacted with the parents of children affected by USH1, and I vividly remember how desperate many of them were for answers — and for hope that one day their babies might regain their hearing and sight. Without knowing which genes were involved, there was little possibility of developing a treatment. But now, gene therapy trials are underway and, within a few years, they may offer cures for children born with this devastating condition. Results like this are deeply motivating, and they remind us that there is still much work to be done.
To succeed in rare disease research, there are a few essential qualities that rise above the rest: intense curiosity, creativity and relentless determination. Scientific research is filled with setbacks, and the best scientists are those who know how to ‘fail well’.
Being a scientist doesn’t really feel like ‘work’ to me — it’s something I genuinely love doing. That said, I do have a life outside the lab with other passions that keep me balanced and energised. One of my biggest interests is cooking. I find a lot of joy in preparing meals and hosting dinner parties, especially when I get to feed people I care about.
Ross’ top tip
Pursue a topic that truly excites you, and embrace the process of ‘failing well’. Setbacks are where the deepest learning and most meaningful breakthroughs begin.
Do you have a question for Ross?
Write it in the comments box below and they will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)

Learn more about researchers’ attempts to treat another genetic disease:
futurumcareers.com/understanding-haemophilia-one-amino-acid-at-a-time





0 Comments