Understanding haemophilia, one amino acid at a time
The hereditary blood disorder haemophilia B can have huge health implications, but it is still far from fully understood. At the University of Washington in the US, Dr Jill M. Johnsen is using the latest DNA sequencing techniques to dig down into the changes to one gene that lead to haemophilia B, examining the role of every amino acid within the protein it encodes. This will create a comprehensive ‘map’ of the gene, leading to an in-depth genetic understanding of the disease.
Talk like a… haematologist
Coagulation factors — a family of proteins in the blood that help form blood clots to stop bleeding
Deep mutational scanning – a method that uses high-throughput DNA sequencing to produce and assess many variants of a protein simultaneously
DNA sequencing — the technique that determines the exact sequence of nucleic acids in a DNA molecule
Haemophilia — an inherited bleeding disorder where the blood does not clot properly. There are different types of haemophilia depending on the coagulation factor affected
In very recent history, determining the sequence of nucleic acids in a gene – a process called DNA sequencing – was a long and laborious process. Now, however, technological advances have made the process astonishingly easier, offering a whole range of opportunities to study genes and genomes at a scale never before possible. “We now have a huge amount of sequencing data to analyse to figure out which changes to DNA sequences are important to health,” says Dr Jill M. Johnsen of the University of Washington. Jill’s lab is working closely with Dr Douglas Fowler from the Department of Genome Sciences to examine how genetic mutations can lead to the bleeding disorder haemophilia B.
Coagulation factor IX
Haemophilia disorders stop blood clotting effectively. When a blood vessel is injured, cells called platelets gather at the site and initiate a complex chain reaction that involves a series of proteins known as coagulation factors. “Haemophilia B occurs when the body can’t make enough coagulation factor IX (nine),” says Jill. “When we sequence the F9 gene responsible for making factor IX in people with haemophilia B, we often find DNA changes.” However, it is difficult to be completely sure that these changes are the root cause of the disease, which has led Jill to undertake a thorough investigation of the F9 gene and the coagulation factor protein it encodes.
“Our project is testing the effects of changing the factor IX protein at every possible amino acid,” says Jill.
“This will tell us which changes affect the level of production of factor IX or lead to the production of factor IX that doesn’t work normally.”
Deep mutational scanning
Typically, testing the impact of a DNA change on a protein involves first creating a specific abnormal gene sequence and testing the effects of this abnormality of gene expression. However, there is a scaling issue with this method. “The factor IX protein is made of 461 amino acids, each of which could be changed by substitution with any one of the other 19 amino acids,” explains Jill. “This means there are 8,759 different possible ways of changing just one amino acid in the protein.” Testing each of these substitutions individually would take years and huge amounts of resources, so it is simply not practical.
With this in mind, Jill worked together with Doug to adopt a different method. “Deep mutational scanning looks at the function of a large number of DNA variants of a protein all at once,” she says. “It’s based on a technique called multiplex assay of variant effect, or MAVE for short.” While MAVEs have been in use for some time, they have only been useful for proteins that stay within cells. Factor IX, however, is secreted from cells into the blood, which is where it performs its coagulation function. Therefore, Jill and her team designed a new MAVE method capable of examining secreted proteins. “This new method should work for many other secreted proteins, too,” says Jill.
The library of variants
Reference
https://doi.org/10.33424/FUTURUM497
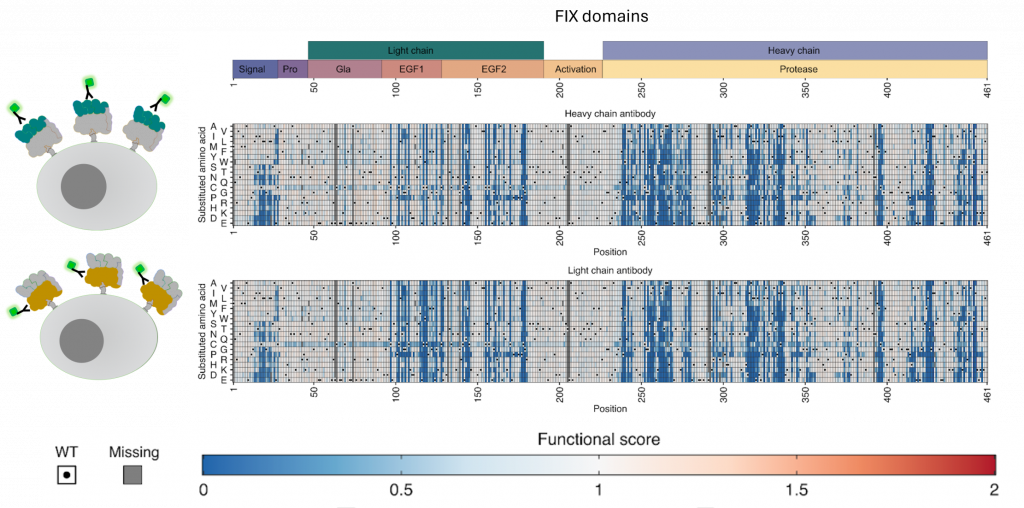
Heatmaps showing the impact of amino acid substitutions on factor IX secretion scores using an antibody to detect the FIX heavy chain (top) and FIX light chain (bottom). Cartoons (left) show how the system works to detect FIX displayed on cells. Blue colors identify amino acid substitutions that are bad for FIX secretion from the cell. Figure credit: Adapted from Popp et al. Manuscript in preparation.
Removing samples from liquid nitrogen storage © Kerry Lannert.
Centrifuging blood samples
© Kerry Lannert.
Jill’s deep mutational scanning method is efficiently analysing the effects of mutating each single amino acid in the factor IX protein through substituting it with each of the other 19 amino acids used in the human body. “This will lead to a comprehensive map of the effects of all possible amino acid changes,” says Jill. “This map will become a useful reference for anyone who wants to know the effect of a particular substitution or pattern of substitutions.” Jill’s team will also enter their results into a database called MAVEdb, which acts as an accessible resource for researchers around the world.
Most importantly, this will help join cause and effect. “If we have a specific DNA change found in a person with haemophilia B, and if we look up that change in our map and find it led to a malfunctioning factor IX in our experiment, we can be sure that this change is causing haemophilia B,” says Jill. The team has now made a map that covers nearly all possible amino acid changes – and they are not stopping there. “Before factor IX is secreted from cells, it is modified in a process called gamma-carboxylation,” explains Jill. “This modification is important for its clotting function, so we are creating a second map that covers how this modification is affected by changes to factor IX.”
Thinking bigger
The series of reactions that leads to effective blood coagulation is complex and involves many different proteins, so the team’s work is not done. “We want to use our MAVE method to test other things that factor IX does, including how it binds to another coagulation factor called factor XI (eleven),” says Jill. “We’ve also started looking at other secreted proteins, such as coagulation factor VIII (eight), which is abnormally low in people with haemophilia A.”
As well as working in the lab, Jill is a practising medical doctor. “I see people living with haemophilia in our clinic and help interpret their genetic findings,” she says. “Being in the clinic helps us design research that better helps people living with haemophilia.” Having this personal experience of haemophilia’s effects on people’s health and well-being lends added motivation to the team’s lab work, as well as making sure that their work will directly benefit those with the disease. “Occasionally, our research has directly helped a person in our clinic,” says Jill.
 Dr Jill M. Johnsen
Dr Jill M. Johnsen
Associate Professor, Division of Hematology and Oncology, Institute for Stem Cell & Regenerative Medicine, Center for Cardiovascular Biology, University of Washington, USA
Field of research: Haematologyy
Research project: Using DNA sequencing to examine the role of every amino acid in protein coagulation factor IX, which affects the development of haemophilia B
Funders: US National Institutes of Health (NIH), Washington Center for Bleeding Disorders (WCBD), Hemophilia of Georgia, Octapharma

About haematology
Haematology is the study of blood. It involves studying the causes, diagnoses, treatments and prevention of blood diseases, such as haemophilia. Like all medical sciences, recent technological advances are accelerating its rate of progress and discovery. Jill explains more about her field.
“Research in this field is rewarding in a number of ways. We get to use cutting-edge technologies to answer interesting questions that have never been tackled before. We are advancing scientific knowledge in an area where people have been suffering, where better diagnoses, predictions and treatments will make a big difference to their well-being.
“Haematology is making unprecedented progress right now. We are generating a wealth of information and diving into it very deeply, down to the impact of individual molecules. Things are moving so fast that it’s difficult to predict what will come next – but it will definitely be even bigger and more exciting, and I would not want to miss out.
“Haematology is about solving puzzles. We are gathering clues to figure out how things work, or don’t work, in the blood. This takes strong critical thinking skills, which doesn’t just involve STEM subjects. For example, a century ago some European royals suffered from a terrible blood disorder, but nobody knew what it was. It was only in 2009, using historical records and DNA sequences of recovered remains, that it was discovered that the royal family had haemophilia B. A diverse array of expertise was needed to solve the case.”
Pathway from school to haematology
Jill emphasises the importance of critical thinking skills for a career in haematology. At school, useful science-based subjects include biology, chemistry, mathematics and physics. Other subjects that can foster critical thinking include history, politics and literature.
At university, relevant degrees include biology, molecular biology, microbiology, biomedical sciences, medicine, genetics and biochemistry.
Explore careers in haematology
The University of Washington runs a number of local and global outreach programmes for high school students and young people.
The University of Washington also offers a variety of research and mentorship opportunities for undergraduates and graduates, including the Hematology Research Training Program and The Institute for Stem Cell & Regenerative Medicine’s Research Experience for Undergraduates.
The American Society of Hematology has a useful career planner, as well as many other resources for people at various career stages.
In the US, a haematologist earns an average of $212,000 per year, according to Salary.com.
Meet Jill
When I entered medical school, I planned to follow a career in infectious diseases. While in residency training, I took care of a young woman with a catastrophic blood clotting disorder. I was angry that we didn’t have enough knowledge to help her – how could we not know? Around that same time, a new heart medicine came out that causes a rare clotting and bleeding complication, but patients were struggling to get a diagnosis. This made me certain that I wanted to work on these puzzles, so I changed my plans and trained in haematology.
Other people have shaped my career hugely. This includes patients, mentors, colleagues and my own trainees. One formative moment was when Doug Fowler and I were put in a room by Debbie Nickerson, a brilliant mentor and scientist, and she told us to work something out. We did – the factor IX project came directly from that incident. And as a fantastic side-effect, we’ve become great friends.
Eureka moments are rare and not like in the movies! My eurekas have come from a slow build-up, before everything snaps into focus. What keeps things exciting are the positive moments when experiments work, new ideas get traction, or we learn something new about a patient’s condition. These make it very worthwhile.
I get to work with fantastic, smart, fun people doing amazing and interesting science. And best of all, it’s all tied to better understanding the diseases that affect people that I care for in the clinic and in my personal life.
I hope to use the programmes we are developing to train more young people. I want to develop structures to support collaboration and sharing amongst researchers. I also want to invite the people affected by the disorders we study into the research process, to help us share our research back with the community.
Jill’s top tip
Don’t worry about reaching a point when you’re ‘done’. For a long time, I kept thinking I would be ‘done’ training. Jobs and pay become real, but you are never done. In this career, you are constantly learning and changing, as the science itself keeps on evolving. The cool part is you get to decide what you are going to be doing.
Do you have a question for Jill and the team?
Write it in the comments box below and Jill and the team will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)
Learn about research happening in paediatric haematology:
www.futurumcareers.com/empowering-people-through-health-education






0 Comments