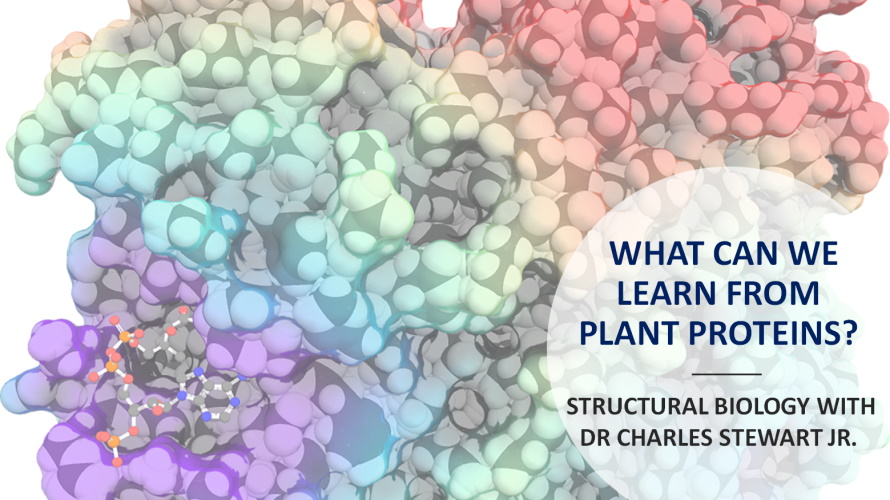
What can we learn from plant proteins?
Metabolism is an essential feature of life. All biological processes, such as photosynthesis, respiration and digestion, depend on various metabolic pathways, which in turn rely upon enzymes to carry out their work. Dr Charles Stewart Jr., of the Macromolecular X-ray Crystallography Facility at Iowa State University in the US, is seeking to improve our understanding of the function of these enzymes by examining their 3D molecular structure
TALK LIKE A STRUCTURAL BIOLOGIST
CATALYSE – to cause or accelerate a chemical reaction
ENZYMES – the proteins responsible for catalysing all chemical reactions in cells
METABOLIC PATHWAY – a series of linked chemical reactions within a cell
METABOLISM – all chemical reactions within a cell that are required to keep it alive
PROTEINS – biological molecules that contribute to almost all activities in an organism
STRUCTURAL BIOLOGY – the study of the 3D structure of biological molecules
X-RAY CRYSTALLOGRAPHY – the use of X-ray radiation to examine the structure and arrangement of molecules inside a crystal
Proteins are the biological molecules that contribute to almost all activities in an organism. From antibodies which fight viruses, to hormones which coordinate biological processes, and to haemoglobin which carries oxygen in the blood, we could not function without proteins. Dr Charles Stewart Jr. of Iowa State University studies the proteins found in plants, specifically the enzymes responsible for plant metabolism. Protein molecules are far too small to be examined by eye under a microscope as most are less than 10 nanometres in diameter (equivalent to 0.00001 mm). In comparison, the average human hair has a diameter of 0.1 mm. This means that you could fit 10,000 protein molecules across the width of a single strand of hair!
HOW DO YOU STUDY SOMETHING SO SMALL?
Charles studies protein molecules using a technique called X-ray crystallography. A beam of X-rays is fired at a rotating protein crystal, and these rays are diffracted (bent) by the protein molecules within it. The intensity and degree of diffraction of the rays are recorded by a detector, which, when combined with the laws of physics underlying X-ray diffraction, enables scientists to build a 3D model of the protein’s molecular structure.
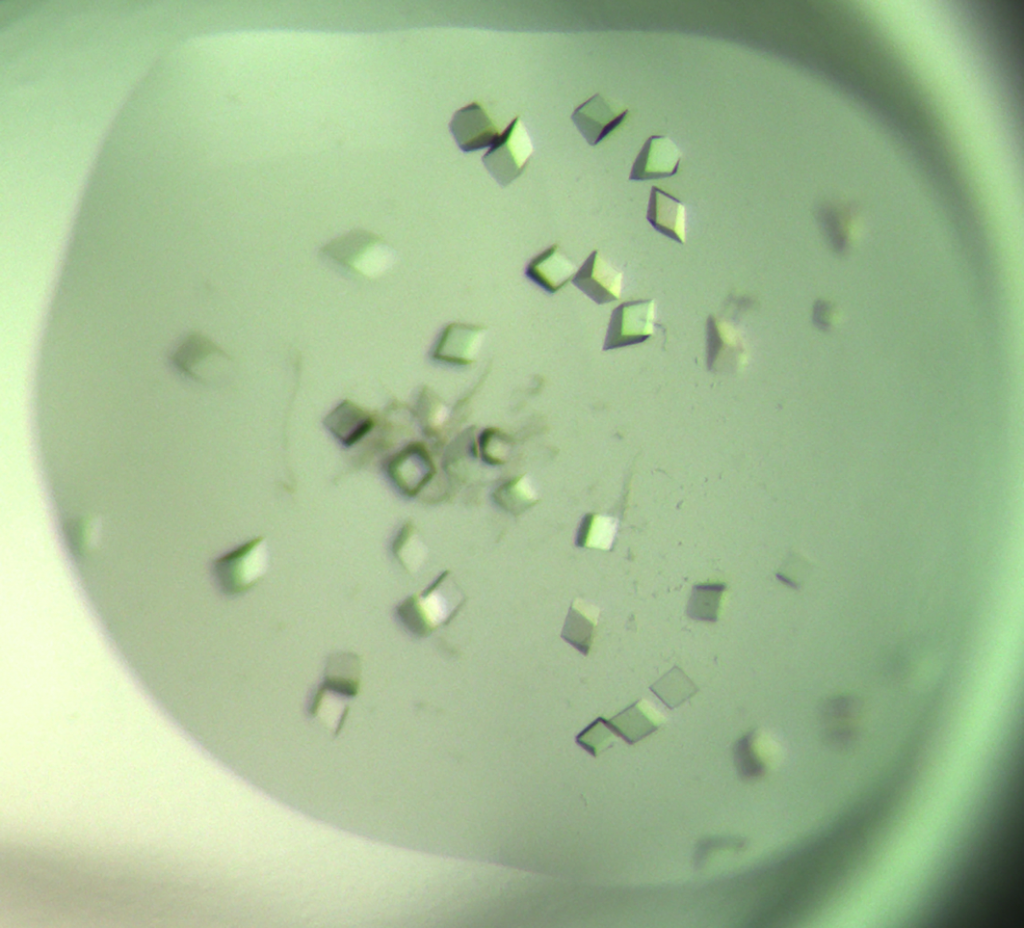
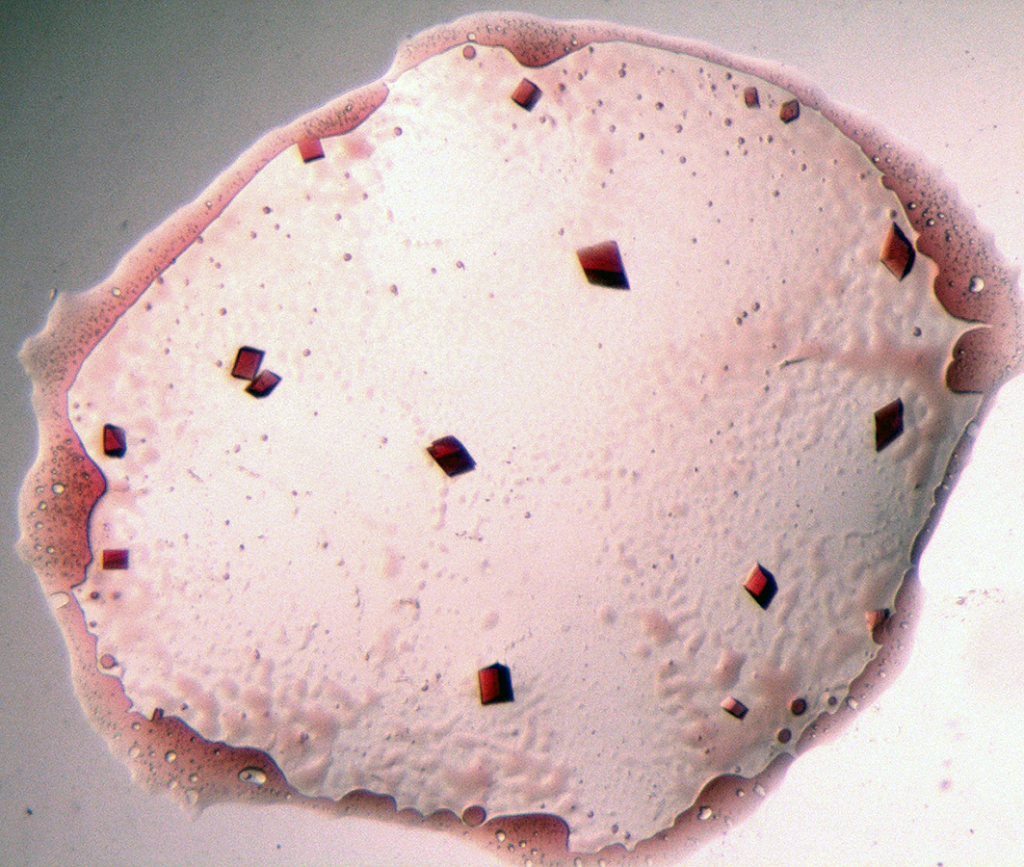
To start the process, Charles must first grow the protein crystal in the laboratory. Unfortunately, protein crystallisation has a high rate of failure, so this is often the most labour-intensive part of the whole procedure. “Although there are general scientific principles to follow, the growing of protein crystals is mostly based on trial-and-error,” explains Charles. It can be hard to predict how the crystallisation experiment will run, so Charles must overcome any technical hurdles as they arise. However, technological advances are helping to address these issues, and robots can now greatly improve the speed of screening different crystallisation parameters.
FINDING BEAUTY IN SMALL THINGS
Charles describes his research as a mix of science and art. He says, “I think that there is an intrinsic beauty to protein crystals and the resultant protein structures, which is often under-appreciated in the scientific literature.” Once he has solved the scientific challenges associated with growing his protein crystals, he has the excitement of discovering the incredible structural details that become visible only with X-ray crystallography. For many of these molecules, Charles is the first person to ever observe what they look like.
One memorable example of this was during his postdoctoral training. After successfully growing new protein crystals, Charles prepared to spend a long night examining them. “Around 2am, my computer monitor started displaying the most beautiful diffraction patterns I had ever seen,” he says. The data he generated that night allowed Charles to view the fine molecular details of a protein, as they had never been seen before, and enabled him to uncover the protein’s 3D structure.
Some of his work today is still based on the hypotheses he developed from his results of that night.
THE MANY BENEFITS OF MEDICINAL PLANTS
Charles’ current research is focusing on two enzymes which catalyse a diverse array of chemical reactions in plants. He is studying polyketide synthases, which are responsible for making defensive molecules, and a methyltransferase, which is thought to transfer a methyl group (a functional group from organic chemistry) onto a nitrogen atom.
The metabolic pathways catalysed by these two enzymes produce tropane alkaloids, a group of molecules made by various plant species that are commonly used in medicines due to their pharmacological properties. Examples include atropine, used to treat pesticide poisoning, hyoscyamine, used to control symptoms of Parkinson’s disease, and scopolamine, used to treat motion sickness, all of which are produced by toxic plants.
However, there are several enzymatic steps of the metabolic pathway that generates tropane alkaloids that are still not understood. It also appears that these enzymes result in different products in different plants, but no one yet knows why this is the case. “I aim to clarify the biosynthetic pathway of tropane alkaloids by focusing on the polyketide synthase and methyltransferase that initiate the process,” explains Charles. “I would like to know not only how these enzymes work, but just as important, what the molecular changes of each enzyme are that determine which products are made in different plants.”
To achieve this, Charles is using X-ray crystallography to discover the molecular structure of multiple enzymes from multiple plant species. Once resolved, he must then establish what role each enzyme is performing in the plant, which is dependent upon its 3D structure. Charles and his collaborators deliberately alter the known structures of these enzymes, generating mutations they think will alter their biochemical activities. By testing whether the mutated enzymes still perform the same functions, they can ascertain exactly which mutations are responsible for causing each enzyme function.
Due to their medicinal value, there is a huge amount of interest in creating tropane alkaloids for pharmaceuticals. The work undertaken by Charles and his colleagues will lay the foundation for bioengineering these molecules, as well as potentially developing new, custom-made proteins that are specifically designed to target medicinal issues.
HAS HE DISCOVERED ANYTHING SO FAR?
Yes! Charles has confirmed that a polyketide synthase is involved in the production of tropane alkaloids, however this particular enzyme uses a different reaction mechanism than common polyketide synthases. He has also discovered that the methyltransferase he has been studying catalyses a biochemical reaction that was previously unknown. In this way, Charles’ research is expanding our understanding of the potential of these enzymes, as well as improving our fundamental knowledge of chemistry itself. “These findings indicate that there are novel enzymes and biochemical pathways still waiting to be discovered,” he explains.
 DR CHARLES STEWART JR.
DR CHARLES STEWART JR.
Manager of the Macromolecular X-ray Crystallography Facility at Iowa State University, USA
FIELD OF RESEARCH: Structural Biology
RESEARCH PROJECT: Investigating the 3D structure of proteins to understand their function
FUNDERS: National Science Foundation (Award 1714148) and National Institutes of Health
Reference
https://doi.org/10.33424/FUTURUM136
CATALYSE – to cause or accelerate a chemical reaction
ENZYMES – the proteins responsible for catalysing all chemical reactions in cells
METABOLIC PATHWAY – a series of linked chemical reactions within a cell
METABOLISM – all chemical reactions within a cell that are required to keep it alive
PROTEINS – biological molecules that contribute to almost all activities in an organism
STRUCTURAL BIOLOGY – the study of the 3D structure of biological molecules
X-RAY CRYSTALLOGRAPHY – the use of X-ray radiation to examine the structure and arrangement of molecules inside a crystal
Proteins are the biological molecules that contribute to almost all activities in an organism. From antibodies which fight viruses, to hormones which coordinate biological processes, and to haemoglobin which carries oxygen in the blood, we could not function without proteins. Dr Charles Stewart Jr. of Iowa State University studies the proteins found in plants, specifically the enzymes responsible for plant metabolism. Protein molecules are far too small to be examined by eye under a microscope as most are less than 10 nanometres in diameter (equivalent to 0.00001 mm). In comparison, the average human hair has a diameter of 0.1 mm. This means that you could fit 10,000 protein molecules across the width of a single strand of hair!
HOW DO YOU STUDY SOMETHING SO SMALL?
Charles studies protein molecules using a technique called X-ray crystallography. A beam of X-rays is fired at a rotating protein crystal, and these rays are diffracted (bent) by the protein molecules within it. The intensity and degree of diffraction of the rays are recorded by a detector, which, when combined with the laws of physics underlying X-ray diffraction, enables scientists to build a 3D model of the protein’s molecular structure.
To start the process, Charles must first grow the protein crystal in the laboratory. Unfortunately, protein crystallisation has a high rate of failure, so this is often the most labour-intensive part of the whole procedure. “Although there are general scientific principles to follow, the growing of protein crystals is mostly based on trial-and-error,” explains Charles. It can be hard to predict how the crystallisation experiment will run, so Charles must overcome any technical hurdles as they arise. However, technological advances are helping to address these issues, and robots can now greatly improve the speed of screening different crystallisation parameters.
FINDING BEAUTY IN SMALL THINGS
Charles describes his research as a mix of science and art. He says, “I think that there is an intrinsic beauty to protein crystals and the resultant protein structures, which is often under-appreciated in the scientific literature.” Once he has solved the scientific challenges associated with growing his protein crystals, he has the excitement of discovering the incredible structural details that become visible only with X-ray crystallography. For many of these molecules, Charles is the first person to ever observe what they look like.
One memorable example of this was during his postdoctoral training. After successfully growing new protein crystals, Charles prepared to spend a long night examining them. “Around 2am, my computer monitor started displaying the most beautiful diffraction patterns I had ever seen,” he says. The data he generated that night allowed Charles to view the fine molecular details of a protein, as they had never been seen before, and enabled him to uncover the protein’s 3D structure.
Some of his work today is still based on the hypotheses he developed from his results of that night.
THE MANY BENEFITS OF MEDICINAL PLANTS
Charles’ current research is focusing on two enzymes which catalyse a diverse array of chemical reactions in plants. He is studying polyketide synthases, which are responsible for making defensive molecules, and a methyltransferase, which is thought to transfer a methyl group (a functional group from organic chemistry) onto a nitrogen atom.
The metabolic pathways catalysed by these two enzymes produce tropane alkaloids, a group of molecules made by various plant species that are commonly used in medicines due to their pharmacological properties. Examples include atropine, used to treat pesticide poisoning, hyoscyamine, used to control symptoms of Parkinson’s disease, and scopolamine, used to treat motion sickness, all of which are produced by toxic plants.
However, there are several enzymatic steps of the metabolic pathway that generates tropane alkaloids that are still not understood. It also appears that these enzymes result in different products in different plants, but no one yet knows why this is the case. “I aim to clarify the biosynthetic pathway of tropane alkaloids by focusing on the polyketide synthase and methyltransferase that initiate the process,” explains Charles. “I would like to know not only how these enzymes work, but just as important, what the molecular changes of each enzyme are that determine which products are made in different plants.”
To achieve this, Charles is using X-ray crystallography to discover the molecular structure of multiple enzymes from multiple plant species. Once resolved, he must then establish what role each enzyme is performing in the plant, which is dependent upon its 3D structure. Charles and his collaborators deliberately alter the known structures of these enzymes, generating mutations they think will alter their biochemical activities. By testing whether the mutated enzymes still perform the same functions, they can ascertain exactly which mutations are responsible for causing each enzyme function.
Due to their medicinal value, there is a huge amount of interest in creating tropane alkaloids for pharmaceuticals. The work undertaken by Charles and his colleagues will lay the foundation for bioengineering these molecules, as well as potentially developing new, custom-made proteins that are specifically designed to target medicinal issues.
HAS HE DISCOVERED ANYTHING SO FAR?
Yes! Charles has confirmed that a polyketide synthase is involved in the production of tropane alkaloids, however this particular enzyme uses a different reaction mechanism than common polyketide synthases. He has also discovered that the methyltransferase he has been studying catalyses a biochemical reaction that was previously unknown. In this way, Charles’ research is expanding our understanding of the potential of these enzymes, as well as improving our fundamental knowledge of chemistry itself. “These findings indicate that there are novel enzymes and biochemical pathways still waiting to be discovered,” he explains.
 DR CHARLES STEWART JR.
DR CHARLES STEWART JR.
Manager of the Macromolecular X-ray Crystallography Facility at Iowa State University, USA
FIELD OF RESEARCH: Structural Biology
RESEARCH PROJECT: Investigating the 3D structure of proteins to understand their function
FUNDERS: National Science Foundation (Award 1714148) and National Institutes of Health
ABOUT STRUCTURAL BIOLOGY
WHY DO WE NEED STRUCTURAL BIOLOGISTS?
A human body is composed of trillions of cells. Each cell contains millions of molecules, and our health depends upon every molecule fulfilling its correct function. Sometimes, molecules within our cells become misshapen, preventing them from interacting as they should, and resulting in diseases.
Structural biology is the study of the 3D structure of biological molecules. Knowledge of how biological molecules are built allows scientists to understand how they act within an organism, and how alterations to their structure cause them to mis-function. Proteins are of special interest to structural biologists, as they are responsible for almost every activity in our body, and each protein has a unique shape related to its specific function. For example, antibodies are Y-shaped to bind to viruses, while enzymes contain pockets that allow them to connect with other molecules. But as soon as the protein’s shape becomes altered, it can no longer perform its job correctly. Cystic fibrosis, Alzheimer’s disease and Parkinson’s disease are all caused by misshapen protein molecules. If structural biologists can determine the structure of these misshapen molecules, then this will improve our understanding of diseases and hopefully help scientists to develop cures.
WHAT OTHER AREAS OF SCIENCE DEPEND UPON STRUCTURAL BIOLOGY?
Structural biology is necessary for any discipline which involves understanding how proteins function at a molecular level. This includes biochemistry, immunology, plant sciences, evolution, microbiology and bioengineering. Many researchers working with Charles are using the Macromolecular X-ray Crystallography Facility to better understand human diseases. For example, Charles is collaborating with Professor Julien Roche who is focused on finding the structure of a protein that predisposes people to mental illness.
Another group is studying the structure of an enzyme from the parasite that causes malaria, which could lead to the basis of antimalarial treatment. Lastly, a group is studying enzymes that are critical for sending signals within cells, which can cause a range of illnesses if they are disrupted. By examining their molecular structure using crystallography, scientists will learn how the enzymes function, which may hopefully lead to the development of cures.
AN INTERESTING CAREER
As structural biology is a multidisciplinary field, Charles works on a wide variety of projects with colleagues from around the world. “Some projects are geared towards fundamental advances in science,” he says, “while others are directly tied to an output that has immediate and obvious benefits to society.”
Charles began his research career studying agricultural biochemistry at Iowa State University. This was followed by a doctorate in plant biology at Cornell University where he investigated how chili peppers make capsaicin, the molecule responsible for their heat, earning him the nickname ‘Dr Pepper’. Alongside his research, he also participated in an agricultural development project, spending two months working with farming communities in northern Ghana. Although this project was unrelated to any research that he had previously performed, Charles was able to apply his scientific knowledge to a new project in a new environment. “It was a great experience and gave me the confidence to go into research areas that were outside my comfort zone.”
Following postdoctoral research positions in California, which saw him develop his skills as a protein crystallographer and begin his studies of polyketide synthases, Charles came full circle to return to Iowa State University as manager of the Macromolecular X-ray Crystallography Facility. “One thing I enjoy about this position is that I am constantly learning,” says Charles. “I work with researchers from different fields, on projects that range from antibodies and human diseases, to proteins underlying agriculturally-important traits, to enzymes being explored to gain fundamental knowledge of chemistry.”
WHAT CHANGES WILL THE FUTURE BRING?
Charles predicts that advances in machine learning and artificial intelligence will transform all science disciplines. He explains, “As machine learning software becomes more user-friendly, scientists from all fields are going to make new discoveries.”
For structural biology, this revolution has already begun. In 2020, a project led by Google, using its artificial intelligence initiative DeepMind, paved the way to overcoming one of the greatest challenges in the field – how to accurately determine the structure of a protein from its amino acid sequence. Future structural biologists will be able to take advantage of these technological innovations to further enhance our understanding of the building blocks of life.
• Charles runs tours of the Macromolecular X-ray Crystallography Facility at Iowa State University for people interested in learning more about the work his team does. If you want to discover what is involved in structural biology, find out if there is a facility near you that you can visit.
• According to PayScale, in the USA a structural biologist can expect an average salary of $105k.
Charles recommends studying the basic science courses such as biology, chemistry, genetics and physics. “But also look for courses that pique your curiosity,” says Charles. “It is hard to predict what courses and experiences are going to fuel your creative flair.”
• A degree in biology or a related subdiscipline is a common route into structural biology. As an interdisciplinary field, other degrees including biochemistry and biophysics can also lead into a career in structural biology.
• To become a structural biologist, you will have to complete a master’s degree or PhD after your initial university studies.
HOW DID CHARLES BECOME A STRUCTURAL BIOLOGIST?
WHAT WERE YOUR INTERESTS AS A CHILD?
I was a curious child. I liked music (singing), math, history and science. I enjoyed the outdoors and re-modelling houses with my father.
WHO OR WHAT INSPIRED YOU TO BECOME A SCIENTIST?
My father, who worked in a factory, stressed the importance of science as a career option. He knew that manufacturing jobs were on the decline in America and foresaw that STEM careers would be growing.
Equally important was my participation in a high school program called Science Bound, operated by Iowa State University, which opened my eyes to the various careers that a major in STEM could provide. I was in the initial class of Science Bound students and had the honor of being the first Science Bound student to graduate from Iowa State University.
WHAT CHARACTERISTICS HAVE MADE YOU A SUCCESSFUL SCIENTIST?
I think having an intrinsic level of curiosity is necessary for success in science. The ability to pay attention to detail and a determination to work through problems are also important.
HOW DO YOU OVERCOME OBSTACLES IN YOUR WORK?
I step back and take a break. When I return to the problem, I will try to break down the obstacle into pieces and figure out which piece is causing the problem. Or I will try to understand if I am making incorrect assumptions. Of course, I will ask others for advice if I feel that they can help me.
WHAT HAVE BEEN YOUR PROUDEST CAREER ACHIEVEMENTS SO FAR?
All the places that I’ve studied or worked have provided invaluable knowledge, wisdom, friendships and memories. I am proud of my work launching the Macromolecular X-ray Crystallography Facility here at Iowa State University. It pushed me out of my comfort zone and allowed me to develop, not only as a scientist, but also as a leader.
WHAT ARE YOUR AMBITIONS FOR THE FUTURE?
As a scientist, I want to keep doing research that furthers our understanding of how nature works. I also want to expand the reach of scientific research to countries and communities that have historically lacked the resources.
As the manager of the Macromolecular X-ray Crystallography Facility, I would like to attract scientists to the facility who may not be experts in crystallography, but who want to use this method to solve their own research problems. Additionally, I would like to develop outreach projects to help young students learn about STEM, not only as a career option, but also as a way to better understand the world we live in.
CHARLES’ TOP TIPS
01 Develop a strong foundation in science.
02 Be willing to learn new things.
03 Find a mentor – someone who you can ask questions of and get feedback from.
Write it in the comments box below and Charles will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)