A new paradigm in antibiotic research
Used for treating bacterial infections, antibiotics are an essential part of modern medicine. However, this crucial treatment option is in jeopardy. Antibiotic resistance – where bacteria become resistant to antibiotics – led to more than 1 million deaths worldwide in 2019. At the University of Basel in Switzerland, Professor Christoph Dehio leads the National Centre of Competence in Research AntiResist, which is applying new research methods to speed up the discovery and development of new antibiotics and complementary anti-infective strategies, thereby helping to reduce the problem of antibiotic resistance.
Talk like an infection biologist
Antimicrobials — a group of medicines that are used to treat infections, including antibiotics, antivirals, antifungals and antiparasitics
Bacterium (plural: bacteria) — a microscopic, single-celled organism
Consortium — a group of people or companies
In vitro — occurring in a controlled environment, such as a test tube or petri dish
In vivo — occurring within a living organism, usually animals, including humans
Microbe — a virus, or a microorganism, such as a bacterium, fungus or parasite
Pathogen — an organism that causes disease
Pneumonia — a life-threatening inflammation of the lungs, typically caused by an infection
Sepsis — when a surge in the body’s immune response triggers inflammation throughout the body, causing a sometimes fatal cascade of organ damage
Antibiotics are medicines that kill or inhibit the growth of harmful bacteria. There are well over 100 antibiotics in total, but penicillin is by far the most prescribed and well known. Antibiotics are used to treat bacterial infections – such as bronchitis, tonsillitis and tuberculosis – but do not work to treat viral infections such as colds, flu or COVID-19.
“Antibiotics are essential for treating severe bacterial infection. Even a simple cut on a finger can develop a serious infection without appropriate attention, leading to the need for antibiotic treatment,” explains Professor Christoph Dehio of the Biozentrum (Bio-centre) at the University of Basel. Antibiotics are indispensable for the treatment of patients with life-threatening infections such as sepsis or pneumonia, and for preventing infections while undergoing surgical procedures, cancer treatments and organ transplants.
Sadly, the effectiveness of antibiotics is under threat. More and more bacteria are evolving to become resistant to antibiotics and fight off the effects of antibiotic treatment. This is known as antibiotic resistance, and it is becoming a greater risk every day.
What is antibiotic resistance?
Antibiotic resistance is a type of antimicrobial resistance (AMR). Although these two terms sound similar, they are not quite the same. AMR is the general term for any microbe resisting any drug that was created to destroy it, including antibiotics, antivirals, antifungals and antiparasitics. Antibiotic resistance is a specific type of AMR in which bacteria resist antibiotics.
How much of a problem is AMR?
AMR has become one of the leading public health threats of the 21st century, with the World Health Organization (WHO) ranking AMR among the top 10 threats to global health. According to the Review on Antimicrobial Resistance* (2016), if more is not done to appropriately address the problem of antibiotic resistance, it could cause 10 million deaths a year by 2050 – more than cancer and diabetes combined.
“Many bacteria can resist one or more antibiotics, meaning that infections caused by those bacteria are hard for doctors to treat,” explains Christoph. While bacteria learn to become resistant to antibiotics naturally over time, poor patient hygiene, lack of access to clean water, over-prescription of antibiotics, and patients taking antibiotics inappropriately can all increase the speed at which antibiotic resistance occurs.
What can be done to tackle AMR?
One important measure to slow down AMR is to use existing antibiotics in the most effective way. This means avoiding unnecessary use of antibiotics and ensuring that whenever an antibiotic is used, it is used with care to minimise the risk of AMR development and spread. This approach, known as antibiotic stewardship, can prolong the period of effective use of an antibiotic for treating patients, but does not resolve the problem of the evolutionary ‘arms race’ ultimately leading to AMR. We urgently need to develop new effective antibiotics that bacteria do not yet know how to resist. However, this solution is not easy.
The first ever antibiotic – penicillin – was discovered by Scottish microbiologist Alexander Fleming in 1928. Over the next 60 years, new antibiotics were discovered regularly. However, the discovery of an antibiotic is not the same as it being available for use. The last new class of antibiotics made available to treat patients was developed over three decades ago, and no more have been developed since. “The lack of new antibiotics is a result of many complex scientific, clinical and economic challenges,” explains Christoph. “With our new approach, we aim to reinvigorate antibiotic discovery and development.”
What is Christoph’s approach?
Christoph is the director of a research consortium called the National Centre of Competence in Research (NCCR) AntiResist, which aims to tackle the rapid and worldwide increase in AMR by developing new, innovative antibiotics. The project is based at the University of Basel, where Christoph works, and includes 30 research groups in Switzerland and one in Israel.
The research project is interdisciplinary, involving doctors, biologists and bioengineers, as well as experts from chemistry, computation and pharmacology. The NCCR AntiResist team also works with pharmaceutical companies that will help produce the new antibiotics that the project discovers.
Which bacteria is the project working on?
NCCR AntiResist is focusing on four difficult-to-treat pathogens: Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Brucella melitensis.
Antibiotic-resistant strains of Escherichia coli (E. coli) have been listed by the WHO as the highest priority for research and development of new antibiotics. “Most E. coli strains are harmless, but some cause serious infections, including food poisoning, septic shock, meningitis, and urinary tract infections (UTIs),” explains Christoph.
The second pathogen, Pseudomonas aeruginosa, is listed by the WHO as a critical priority. It is frequently found in serious infections such as pneumonia, sepsis, burns, and wound infections.
NCCR AntiResist’s third pathogen of focus, Staphylococcus aureus, is causing an increasing number of deaths worldwide due to pneumonia, and skin and soft-tissue infections.
The fourth pathogen, Brucella melitensis, causes the chronic debilitating disease brucellosis. People most commonly get this disease by eating unpasteurised dairy products that come from infected sheep, goats, cows or camels.
The AntiResist team is targeting these four bacterial pathogens as their resistance to antibiotics makes them the most threatening to global health. “Our ultimate goal is to use our ground-breaking approach to find new types of antibiotics and other innovative anti-infective therapies that are able to treat infections caused by these bacteria,” Christoph explains.
How does the project work?
The AntiResist project has multiple phases which all require close collaboration between medical doctors, biologists and bioengineers.
“Recent attempts to discover new type of antibiotics in the laboratory – in vitro – struggle when clinically tested on people,” says Christoph. To avoid this, the NCCR project performs its studies both in vivo and in vitro.
Firstly, medical doctors within the AntiResist team gather samples of bacteria from infected patients. This means the process starts by working directly with human tissue. “Studying microorganisms inside human tissue – in vivo – enables us to learn more about bacterial behaviour and its interactions inside the infected patient,” explains Christoph.
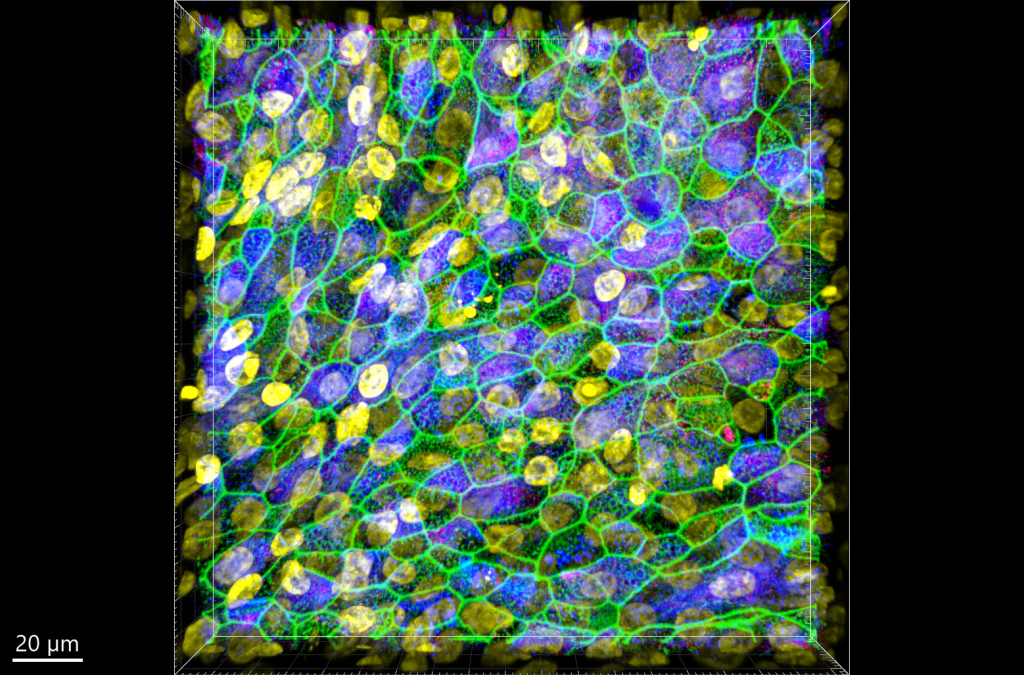
After sampling tissue from the patient, samples are transferred from hospitals to laboratories, where they are studied by biologists. “Biologists analyse the samples using highly sensitive, state-of-the-art analytical methods,” explains Christoph. “The results are then used to develop micro-tissue models that simulate the conditions in the human tissue as accurately as possible. This is the laboratory – in vitro – phase.”
These micro-tissue models are developed by growing a tissue inside a culture dish in conditions that mimic the environment of the human body. After the microtissue is fully developed, the biologists introduce bacterial cells to the dish and observe what happens, how the bacteria move and replicate, how they infect the tissue and how the tissue might defend itself.
Once the biologists are satisfied with how these models are working, they collaborate with bioengineers to take the first steps towards discovering new antibiotic treatments.
What has the team found so far, and what is next?
The AntiResist team is coming to the end of the first phase of its research, during which it has gathered human tissue samples, analysed them in the laboratories, and begun to create the in vitro models.
During the second phase of the project, the team will identify the specific ways that each tissue was infected by bacteria, and the mechanisms involved. “From there, we will be able to pinpoint vulnerable factors of the bacteria to target,” says Christoph. This means that the NCCR team and their collaborators in the private sector will use the developed models and their knowledge on vulnerable bacterial factors to identify novel antibiotic compounds and principles.
In the third, final, phase, the goal of NCCR AntiResist is to develop these antibiotic compounds and principles further to eventually move the research into clinical trials, which is one step away from introduction into clinical practice. Keep your eyes peeled to see what treatment options we might have in the future!
* amr.review.org
 Professor Christoph Dehio
Professor Christoph Dehio
Biozentrum, The Center for Molecular Life Sciences, University of Basel, Switzerland
Fields of research: Molecular Microbiology, Infection Biology, Infectious Diseases, Bioengineering
Research project: Identifying new strategies to fight bacterial infections
Funder: Swiss National Science Foundation (SNSF)
Reference
https://doi.org/10.33424/FUTURUM507
About infection biology and antibiotic research
Spread across 31 research groups, the NCCR AntiResist team consists of biologists, chemists, clinicians, bioengineers, computer scientists and physicists. The range of expertise within the team highlights the complexity of the research challenge and the multidisciplinary approach the team is taking to tackle it. It also shows that there is not one set career pathway you must follow to contribute to antibiotic research; many research interests could enable you to do so. From clinical studies looking at how pathogens ‘behave’ in an infected patient to modelling how new antimicrobials could work, from developing technologies that enable genetic analysis of pathogens to developing sophisticated databases to store and interpret the huge amount of data such research generates, the career opportunities offered by infection biology and antibiotic research are wide and varied.
The ambitions of NCCR AntiResist highlight the innovative research required for progress in this field, and Christoph and the team believe that the next generation of researchers have an important part to play in this. “We hope that young scientists who want to make a difference in the AMR field will learn a new research approach to tackle resistances,” explains Christoph. He emphasises that the future of research in this vital field is multi-faceted and multi-disciplinary: “Being able to gain insights from other fields of expertise can be inspiring to your own work and paves the way for great advances in knowledge.”
To ensure it is preparing a new generation of scientists to keep moving research forward, the NCCR AntiResist team is committed to giving its students opportunities to gain research experience outside the classical disciplinary route. “As part of our work, we ensure that our Master’s and PhD students are exposed to different disciplines, so that they are prepared and qualified to carry our approach into future scientific discoveries,” says Christoph. “Our consortium offers PhD fellowships which give students the opportunity to choose between biology, clinical and engineering laboratories in their first year. These students work across at least two disciplines, so that they gain expertise in a truly interdisciplinary context.”
Antimicrobial resistance will be an ongoing challenge, and Christoph and the NCCR AntiResist team are ensuring that scientists and researchers – both now and in the future – are able to meet it head on.
Pathway from school to infection biology and antibiotic research
• The NCCR AntiResist researchers work across clinical, biological and engineering fields, representing a wide variety of academic backgrounds. As well as biology, subjects such as chemistry, computing and engineering-related subjects are relevant. If you look at the ‘People’ pages on the AntiResist website, you can see the detailed CVs of the team’s principal investigators and the variety of qualifications and expertise within the consortium.
• “At the Biozentrum (Bio-centre) in Basel, there are regular public lectures called ‘Einblicke’ open to anyone who wants to attend,” says Christoph. The calendar of events starts afresh every September.
• As the NCCR AntiResist team highlights, research in this area crosses countries, as well as disciplines. Christoph says, “Be proactive and start searching online to find out what work is going on in your country. Search terms such as ‘microbiology’, ‘microbes’, ‘infection biology’, ‘drug development’, ‘antibiotic research’ and ‘antibiotic resistance’. There is a wealth of resources to explore.”
• To find more challenging content and scientific papers, Christoph advises searching on Google Scholar, using terms such ‘AMR’ or ‘antibiotic research’.
• “The ReACT toolbox is a very useful site for learning about antibiotic resistance, the latest scientific research and ways to raise awareness,” says Christoph.
Explore careers in infection biology and antibiotic research
• The PhD students who work with Christoph come from a wide range of backgrounds, from medicine to data science, engineering to biochemistry and microbiology, which highlights the scope of disciplines and career paths linked to infection research. Visit the NCCR AntiResist website, where you can read about the different routes the PhD fellows took before joining NCCR AntiResist.
• In the UK, Prospects provides information on a range of careers that could see you playing a role in infection research. For example, in data science, biochemistry, microbiology and medicine.
• You can read about how other researchers – from varying fields – are studying antibiotic resistance and the career paths they have taken in other Futurum articles. For example, from the perspective of a chemical microbiologist, a chemical engineer and medical anthropologists.
• The American Society for Microbiology hosts a podcast, Editors in Conversation, which highlights different pathways to follow careers in antimicrobial research.
Meet Christoph
I grew up on a farm and have always been fascinated by nature in all its manifestations and interested in exploring its mysteries. In my final year at school, I visited Dietrich von Holst, a distant relative who, at the time, was Professor of Animal Physiology at the University of Bayreuth in Germany. His physiological and behavioural studies of stress in tupaias (tree shrews) fascinated and inspired me to take the academic route and become a scientist and researcher.
Serendipity has played a significant role in shaping my career, but I am also grateful for the guidance and support of mentors who have provided me with the necessary freedom to grow into an independent scientist. Their mentorship has been instrumental in helping me navigate the academic path and advance through the ranks.
There have been eureka moments at every stage of my academic career. For example, as a young postdoc at Institut Pasteur, I discovered that infection of human cells by the bacterial pathogen Shigella triggers the activity of a protein involved in cancer (a so-called proto-oncogene). As is the case in every scientist’s career, these moments are few and far between, but when they do happen, they drive the urge for research progress.
My success as a scientist and in overcoming obstacles stems from my curiosity, perseverance, excitement for research, independence and willingness to collaborate.
I am particularly proud of several things in my career. Firstly, I am proud that I – together with my research team – pioneered and advanced molecular studies on the emerging pathogen Bartonella, which led to a detailed understanding of the molecular infection process and offered interesting targets for treatment and prevention. Secondly, that a sizeable number of scientists trained in my lab are now leaders in academic research and industry. Thirdly, with the support of my colleagues and the Swiss National Science Foundation, we launched the NCCR AntiResist project, which could significantly change the way we discover and develop anti-infectives in the future.
Christoph’s top tip
Do what you like best, and do it with full dedication.
Do you have a question for Christoph?
Write it in the comments box below and Christoph will get back to you. (Remember, researchers are very busy people, so you may have to wait a few days.)
Read about how bacteria can pass on ‘memories’ to their offspring:
www.futurumcareers.com/remembering-resistance-nongenetic-memory-in-bacteria














0 Comments